Sequana Medical NV announces that it has completed implanting patients in POSEIDON, the North American pivotal study to support regulatory approval of the alfapump system in the U.S. and Canada, for the treatment of recurrent or refractory ascites due to liver cirrhosis.
- Reporting of primary endpoint on track for Q4 2022
- FDA regulatory submission on track for mid-2023
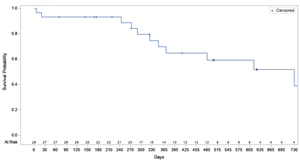
- 70% survival of POSEIDON Roll-In patients at one year post-implantation1 compares favourably to published literature in this high-risk patient population
GHENT, Belgium, April 05, 2022 (GLOBE NEWSWIRE) -- Sequana Medical NV (Euronext Brussels: SEQUA) (the “Company” or “Sequana Medical”), an innovator in the treatment of diuretic-resistant fluid overload in liver disease, malignant ascites, and heart failure, today announces that it has completed implanting patients in POSEIDON, the North American pivotal study to support regulatory approval of the alfapump system in the U.S. and Canada, for the treatment of recurrent or refractory ascites due to liver cirrhosis.
Of the 71 patients enrolled in the Pivotal Cohort, 40 patients have been implanted with the alfapump. Pivotal patients completing the six-months post-implantation period will be included in the primary efficacy endpoint analysis, and reporting of these data is planned for Q4 2022. A further 29 patients from the Roll-In Cohort were also implanted with the alfapump and will be included in the overall safety analysis.
Importantly, a preliminary interim analysis1 of patient survival following alfapump implantation in the Roll-In Cohort indicated a mean survival probability of 70% at 12 months. This compares favourably with the published literature reporting a survival rate for refractory ascites patients of only 50% at 12 months.2
“Completing alfapump implantations is an important milestone for our POSEIDON study given the uncertainties of hospital resource availability that still exist due to COVID, and enables us to report the primary endpoint data before the end of this year as planned,” commented Ian Crosbie, Chief Executive Officer of Sequana Medical. “Given the strong efficacy results we reported in last year’s interim analysis, we are confident that these implanted patients in the Pivotal Cohort will give us sufficient statistical power for the primary efficacy endpoint. For our safety evaluation, we are including the data from the Roll-In patients which will bring the total number in that assessment to approximately 70.”
“We are encouraged by our preliminary interim review of patient survival with 70% of the POSEIDON Roll-In patients alive at 12 months post-implantation,” added Dr. Gijs Klarenbeek, Senior Medical Adviser of Sequana Medical. “We believe that this survival data combined with the dramatic reduction in the rate of therapeutic paracentesis and the clinically relevant improvement in quality of life in the interim analysis of the first 26 Roll-in patients suggest that the alfapump is a highly attractive treatment option for this patient group that has been overlooked for too long.”
Kaplan Meier – Preliminary survival rate analysis of Roll-In Cohort, dated 25 March 2022:
https://www.globenewswire.com/NewsRoom/AttachmentNg/1a65f324-c436-4786-8089-3c891f88037b
Summary of the 2nd interim analysis of Roll-In Cohort reported in July 2021
In July 2021, the Company reported the second interim analysis on 26 patients from the Roll-In Cohort, reaffirming the previous positive efficacy results of the alfapump and providing longer-term evidence of the reduction in therapeutic paracentesis and continued improvements in quality of life. Data from this Roll-In Cohort substantially exceeded the pre-defined requirements of the primary endpoints as defined for the Pivotal Cohort in the study3, demonstrating: (i) over 90% reduction in mean frequency of therapeutic paracentesis (TP) versus baseline, (ii) all patients having at least a 50% reduction in mean frequency of TP per month versus baseline, (iii) clinically important improvement in quality of life maintained even up to 12 months post-implantation, and (iv) safety profile in line with expectations.
For more information, please contact:
Sequana Medical
Lies Vanneste
Director Investor Relations
Tel: +32 498 05 35 79
Email: IR@sequanamedical.com
LifeSci Advisors
Guillaume van Renterghem
Tel: +41 76 735 01 31
Email: gvanrenterghem@lifesciadvisors.com
About the POSEIDON study
POSEIDON is a single-arm, open-label and within subject cross-over study of the alfapump in patients with recurrent or refractory ascites due to liver cirrhosis and is being conducted in approximately 20 centres across the U.S. and Canada. All patients have been enrolled in the study and implanted with the alfapump. Patients enrolled in the Pivotal Cohort entered into a three-month pre-implant observation period before they were implanted with the alfapump. The study allowed to enrol additional patients in a Roll-In Cohort to ensure new centres were experienced with the alfapump implantation prior to enrolling patients in the Pivotal Cohort.
The primary effectiveness outcomes of the study include the proportion of patients with a 50% reduction in the overall average frequency of therapeutic paracentesis per month in the post-implant observation period (month four to month six post-implantation) as compared to the pre-implant observation period. The primary safety endpoint is the rate of alfapump-related re-interventions adjudicated by the Clinical Events Committee. Patients will be followed for up to two years for analysis of secondary outcome measurements including safety (device and/or procedure-related adverse events), quality of life (assessed by general SF36 as well as disease-specific Ascites Q questionnaires), nutritional status, health economics and overall survival. For more information about the study, please visit clinicaltrials.gov (NCT03973866).
About Sequana Medical
Sequana Medical is a commercial stage medical device company utilizing its proprietary alfapump® and DSR® (Direct Sodium Removal) technologies to develop innovative treatments for fluid overload in liver disease, malignant ascites and heart failure where diuretics are no longer effective. Fluid overload is a frequent complication of many large diseases — including advanced liver disease driven by NASH (non-alcoholic steatohepatitis)-related cirrhosis and heart failure — with diuretic resistance being widespread. The U.S. market for the alfapump resulting from NASH-related cirrhosis is forecast to exceed €3 billion annually within the next 10-20 years. The heart failure market for DSR and the alfapump DSR® is estimated to be over €5 billion annually in the U.S. and EU5 by 2026.
The alfapump is Sequana Medical’s unique, fully implanted wireless device that automatically pumps fluid from the abdominal cavity into the bladder, where it is naturally eliminated through urination. DSR is Sequana Medical’s proprietary approach to managing sodium and fluid overload through use of a sodium-free infusate administered into the abdominal cavity.
In the U.S., the Company’s key growth market, the alfapump has been granted breakthrough device designation by the FDA for recurrent or refractory ascites due to liver cirrhosis. Interim data from the ongoing North American pivotal study (POSEIDON) showed positive outcomes against all primary endpoints and a rapid and persistent clinically important improvement in quality of life. All patients have been implanted with the alfapump and primary endpoint reporting is planned for Q4 2022. This study is intended to support a future marketing application of the alfapump in the U.S. and Canada. In Europe, the alfapump is CE-marked for the management of refractory ascites due to liver cirrhosis and malignant ascites and is included in key clinical practice guidelines. Over 900 alfapump systems have been implanted to date.
Sequana Medical has combined its proven alfapump and proprietary DSR therapy, and is developing the alfapump DSR, a breakthrough approach to fluid overload due to heart failure. RED DESERT demonstrated that repeated DSR therapy in diuretic-resistant heart failure patients is able to manage their fluid and sodium balance, improve their cardio-renal status and restore their diuretic response for months post-treatment. Interim results from the ongoing SAHARA DESERT study in decompensated heart failure patients indicate that repeated DSR therapy can safely, effectively and rapidly eliminate persistent congestion and restore euvolemia, together with considerable benefit in cardio-renal status and a dramatic improvement in diuretic responsiveness. Reporting of top-line data is planned for H2 2022.
Sequana Medical is headquartered in Ghent, Belgium. For further information, please visit www.sequanamedical.com.
Important Regulatory Disclaimers
The alfapump® system is not currently approved in the United States or Canada. In the United States and Canada, the alfapump system is currently under clinical investigation (POSEIDON Study) and is being studied in adult patients with refractory or recurrent ascites due to cirrhosis. For more information regarding the POSEIDON clinical study see www.poseidonstudy.com. The DSR® therapy is still in development and it should be noted that any statements regarding safety and efficacy arise from ongoing pre-clinical and clinical investigations which have yet to be completed. The DSR therapy is not currently approved for clinical research in the United States or Canada. There is no link between the DSR therapy and ongoing investigations with the alfapump system in Europe, the United States or Canada.
Note:alfapump® is a registered trademark. DSR® is a registered trademark in Australia, the Benelux, the EU, United Kingdom, Hong Kong, Israel, Norway, and Switzerland. alfapump DSR® is a registered trademarks in Australia, the Benelux, China, the EU, United Kingdom, Hong Kong, Israel, New Zealand, and Norway.
Forward-looking statements
This press release may contain predictions, estimates or other information that might be considered forward-looking statements. Such forward-looking statements are not guarantees of future performance. These forward-looking statements represent the current judgment of Sequana Medical on what the future holds, and are subject to risks and uncertainties that could cause actual results to differ materially. Sequana Medical expressly disclaims any obligation or undertaking to release any updates or revisions to any forward-looking statements in this press release, except if specifically required to do so by law or regulation. You should not place undue reliance on forward-looking statements, which reflect the opinions of Sequana Medical only as of the date of this press release.
1 Date of analysis 25 March 2022
2 Biggins et al., Hepatology, Vol. 74, No. 2, 2021, AASLD Practice Guidance; Moreau R et al., Liver International 2004: 24: 457-464
3 Pre- and post-implant periods for this analysis of the Roll-In Cohort differ from those that will be used for the Pivotal Cohort analysis