New Dry Eye and MIGS Entrants will be Prominently Featured in Product Demonstrations and Events at Upcoming Industry Shows
New Dry Eye and MIGS Entrants will be Prominently Featured in Product Demonstrations and Events at Upcoming Industry Shows
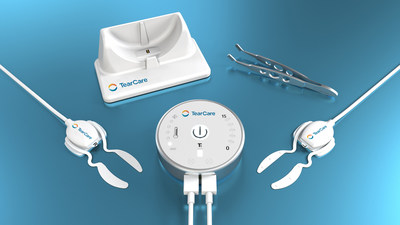
MENLO PARK, Calif., April 30, 2019 /PRNewswire/ -- Sight Sciences, Inc., a growth-stage medical device company transforming the two fastest growing segments in ophthalmology and optometry – dry eye and glaucoma – today announced the official commercial launch of TearCare® and the introduction of an aesthetically and ergonomically enhanced OMNI® Surgical System (which was initially launched in February 2018).
The rollout of the new devices will include product demonstrations, presentations and events at a number of the eye care industry's upcoming premier shows, including:
- Vision Source Exchange, held May 1-4 in Tampa, FL, at booth #1129.
- American Society of Cataract and Refractive Surgery and American Society of Ophthalmic Administrators (ASCRS/ASOA) annual meetings, held May 3-7 in San Diego, CA, at booth #1307.
- American Optometric Association (AOA) annual meeting, held June 19-23 in St. Louis, MO, at booth #1215.
"We are excited to launch these two innovative products that can transform the way surgeons and eye care professionals target the underlying cause of two of the world's most prevalent eye diseases. We have been thrilled with the market receptivity to our first-generation OMNI® system, which we have been selling since early 2018 through our world class surgical commercial team built from the ground up by Shawn O'Neil, our Chief Commercial Officer. Given Shawn's long history of developing and leading high-performance teams in both surgical devices and vision care, we are excited to replicate this success with the commercial launch of TearCare®," said Paul Badawi, Founder and CEO of Sight Sciences.
"First and foremost, our surgical and vision care teams fully appreciate the clinical value of the groundbreaking products we are bringing to our MD and OD customer bases. We have built a highly robust and pedigreed commercial force comprised of two discrete, specialized sales and marketing teams for both the Dry Eye (TearCare®) and MIGS (OMNI®) practice areas. We are well-positioned to further accelerate the market adoption of OMNI® in the coming months as we roll out our next generation product and to execute a best-in-class commercial launch for TearCare®. As we penetrate and expand these two substantial, rapidly growing markets over the coming years, our goal is to be amongst the top performing companies in ophthalmology as well as MedTech more broadly," said Shawn O'Neil, Chief Commercial Officer of Sight Sciences.
TearCare®
TearCare® is the world's first and only wearable device providing a personalized open-eye experience. The TearCare® device allows the patient's eyes to remain open and blinking during the procedure where localized heat application is needed. Soft, flexible thermal devices conform to the eyelids to deliver a therapeutic level of heat for a specific period of time to soften and liquefy meibum, the oily coating on the eye surface which prevents tear film evaporation. TearCare®'s innovative, "equipment-light" product design and the intuitive procedure it facilitates create a highly attractive clinical and economic model for eye care providers. Customers interested in purchasing TearCare® will have the opportunity to order the product at the Sight Sciences' booths at the industry events detailed herein.
"With a better understanding of the difference between aqueous deficiency and excessive evaporation as root causes of dry eye, and the rapidly growing number of evaporative dry eye disease cases, eye care professionals now more than ever before are looking for a new alternative for their evaporative dry eye patients," said O'Neil. "We are pleased to offer them TearCare®, a first-of-its-kind device that delivers on our promise to develop innovation that targets the underlying disease state of the condition."
TearCare® Clinical and Scientific Studies
A single center, prospective pilot study published in the April 2018 and January 2019 issues of Clinical Ophthalmology provided preliminary evidence that an initial treatment with the TearCare® System as well as a re-treatment after six months delivered a sustained and statistically significant improvement in the signs and symptoms of dry eye disease at the primary end point time of 4 weeks and at each follow-up visit through 12 months. The TearCare® System is a tool that is indicated for the application of localized heat, including in meibomian gland dysfunction, dry eye and blepharitis. Currently, Sight Sciences is enrolling participants in a large, prospective, multi-center, randomized controlled trial.
Next Generation OMNI® Surgical System
Commercially launched in early 2018, the OMNI® Surgical System combines the distinct functions of its two predicate devices – TRAB®360 for trabeculotomy and VISCO360® for transluminal viscoelastic delivery – into one device. OMNI® enables the targeting of all three sources of resistance in the conventional outflow pathway (trabecular meshwork, Schlemm's canal, and collector channels) with a single device and single surgery.
The next generation OMNI System features improvements in device preparation, handling and ergonomics, and aesthetics. OMNI®'s enhanced design was created based on observations of its use in the broader market as well as specific guidance from world-renowned glaucoma specialist, surgical device innovator, and Chief Medical Officer of Sight Sciences, Dr. Reay Brown. This version of OMNI® will be introduced through demos at upcoming industry events and commercially available to surgeons after completion of their training on the device.
"While surgeons and glaucoma specialists were happy with OMNI's unique ability to facilitate two distinct and comprehensive procedures within the conventional outflow pathway, we saw an opportunity to improve the device preparation, ergonomics, and the look and feel of the device with the goal of improving the overall experience for the surgeon. One of my personal goals at Sight Sciences is to drive innovation in the effort to make OMNI the MIGS of choice for all surgeons," said Dr. Brown.
OMNI® Surgical System's Clinical and Scientific Studies
Published in the January 2019 issue of Clinical Ophthalmology, a single center retrospective study of glaucoma surgery patients showed that ab-interno trabeculotomy using one of Sight Sciences' OMNI® Surgical System's predicate devices (TRAB®360) provided a favorable safety profile and substantial long-term reductions in both intraocular pressure (IOP) and IOP-lowering medications.
Additionally, enrollment has begun in the Gemini clinical study, a Sight Sciences-sponsored, prospective, multi-center study to evaluate the safety and efficacy of the OMNI® Surgical System in performing two sequential Micro-Invasive Glaucoma Surgery procedures – transluminal viscoelastic delivery and trabeculotomy. The study will enroll 130 subjects from 10 to 15 medical centers in the United States. Participants will be followed for one year with an interim analysis performed at six months.
"For the millions of patients who suffer from glaucoma and dry eye disease, Sight Sciences is committed to focusing on the two largest unmet needs in eye care," said Dr. Brown. "It all starts with building a compendium of scientific evidence to help inform eye care professionals' clinical decisions and help them select technologies that are best suited for their individual patients."
About TearCare®
The TearCare® System is indicated for the application of localized heat when the current medical community recommends the application of a warm compress to the eyelids. Such applications would include Meibomian Gland Dysfunction (MGD), Dry Eye, or Blepharitis.
About OMNI® Surgical System
The OMNI® Surgical System is a manually operated device for delivery of small amounts of viscoelastic fluid, for example Healon® or HealonGV® from Abbott Medical Optics (AMO), Amvisc® from Bausch & Lomb, or PROVISC® from Alcon, during ophthalmic surgery. It is also indicated to cut trabecular meshwork tissue during trabeculotomy procedures.
The OMNI® System should not be used in cases where there is insufficient visualization of the anterior chamber. The following conditions may prohibit sufficient visualization required for safe and successful cannula and microcatheter placement: corneal edema, corneal haze, corneal opacity, or any other conditions that may inhibit surgeon view.
The TRAB®360 Trabeculotomy System is a trabeculotome, a manual surgical ophthalmic instrument used to mechanically cut trabecular meshwork.
The OMNI® Surgical System and TRAB®360 Trabeculotomy System are tools, not treatments, and are indicated for use as specified above; they are not specifically cleared by the FDA to lower intraocular pressure in patients with open angle glaucoma.
About Sight Sciences
Founded in 2011, Sight Sciences is a commercial-stage medical device company dedicated to the development of intelligently designed and engineered products that address the underlying physiology of ophthalmic diseases. The company's surgical glaucoma product portfolio features the OMNI® Surgical System. Its non-surgical dry eye product portfolio consists of TearCare® for ophthalmologists and optometrists. For more information, please visit sightsciences.com.


![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/sight-sciences-announces-official-launches-of-tearcare-and-the-enhanced-next-generation-omni-system-300840287.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/sight-sciences-announces-official-launches-of-tearcare-and-the-enhanced-next-generation-omni-system-300840287.html
SOURCE Sight Sciences




