Smith+Nephew, the global medical technology company, announces it will showcase its latest Sports Medicine procedural innovations for joint repair and arthroscopic enabling technologies during the American Academy of Orthopaedic Surgeons 2023 Annual Meeting being held in Las Vegas, NV.
LONDON, March 8, 2023 /PRNewswire/ -- Smith+Nephew (LSE:SN) (NYSE:SNN), the global medical technology company, today announces it will showcase its latest Sports Medicine procedural innovations for joint repair and arthroscopic enabling technologies during the American Academy of Orthopaedic Surgeons 2023 Annual Meeting being held in Las Vegas, NV. These include:
UltraTRAC™ QUAD ACL Reconstruction Technique: New technologies combine to deliver an advanced, comprehensive procedural solution for surgeons who want to expand their ligament graft options and maximize the variety of patients they can accommodate. Together, the new QUADTRAC™ Quadriceps Tendon Harvest Guide System, X-WING™ Graft Preparation System and ULTRABUTTON™ Family of Adjustable Fixation Devices provide a controlled and reproducible technique to harvest only the desired quadriceps tendon tissue.1-3 Quadriceps ACL reconstruction is a growing procedure that offers many advantages including a lower risk of anterior knee pain4-6 and predictable, larger graft diameter4,7,8 compared to bone-tendon-bone or hamstring autograft options respectively.
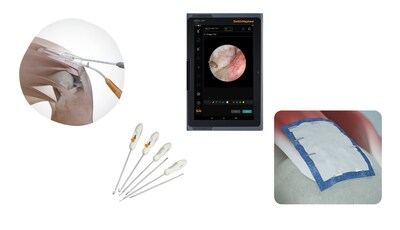
INTELLIO™ Tablet: Helping streamline your procedures with simple, centralized connectivity in the palm of your hand - the INTELLIO Tablet is a medical-grade device designed for use with Smith+Nephew’s INTELLIO Connected Tower. Supporting both wireless and tethered connectivity, the INTELLIO Tablet increases users’ ability to control and adjust the LENS 4K Surgical Imaging System, DYONICS™ POWER II Control System, WEREWOLF™ COBLATION™ System and the DOUBLEFLO™ Inflow/Outflow Pump.
CAP-FIX™ Capsular Management Family: A comprehensive range of products designed to maximize the efficiency of capsular management procedures. The CAP-FIX Suture Passer reduces the number of steps required for capsule closure, compared to suture shuttling,9 and is optimized for sharpness, strength, trajectory and suture throw.9-10 CAP-FIX Blades are offered as curved or straight with a choice of a pencil grip or a handle for increased leverage. Each blade has a consistent sharpness to cut through hip capsular tissue.11 From open to close, the CAP-FIX Capsular Management Family and the full portfolio of hip preservation products encourages surgeons to “Go Beyond the Repair.”
REGENETEN™ Bioinductive Implant: Supports the body’s natural healing response to facilitate new tendon-like tissue growth and changes the course of rotator cuff tear progression.12-17 The implant is about the size of a postage stamp and has been shown to be completely resorbed within six months.*14,17 In addition to rotator cuff repair, the REGENETEN Bioinductive Implant can be used for hip repair as well as Achilles repair and reconstruction.
To learn more about these ground-breaking Sports Medicine innovations, please stop by the Smith+Nephew booth (#2021) during AAOS 2023
References
*On human biopsy (n=1) and in-vivo sampling
1 Smith+Nephew 2022. QUADTRAC Marketing Claims. Internal Report. 15011572 Rev A.
2 Smith+Nephew 2022.TRAC-Cutter Push-Pull Cut Force DV Protocol. Internal Report. 15011552 Rev A.
3 Smith+Nephew 2022.QUAD-Cutter Force to Cut Design Verification. Internal Report. 15011559 Rev A.
4 Buerba RA, Boden SA, Lesniak B. Graft Selection in Contemporary Anterior Cruciate Ligament Reconstruction. JAAOS: Global Research and Reviews. 2021;5(10).
5 Lund, Bent et al. “Is Quadriceps Tendon a Better Graft Choice Than Patellar Tendon? A Prospective Randomized Study.” Arthroscopy 2014; 30(5): 593-598..
6 Malinowski K, Paszkowski J, Mostowy M, Goralczyk A, Laprade RF, Hermanowicz K. Quadriceps Tendon-Bone Full-Thickness Autograft: Reproducible and Easy Harvesting Technique Using Simple Surgical Tools. Arthroscopy Techniques. 2021;10(4).
7 Todor A, Nistor DV, Caterev S. Clinical outcomes after ACL reconstruction with free quadriceps tendon autograft versus hamstring tendons autograft. A retrospective study with a minimal follow-up two years. Acta Orthop Traumatol Turc. 2019;53(3):180-183.
8 Runer A, Csapo R, Hepperger C, Herbort M, Hoser C, Fink C. Anterior Cruciate Ligament Reconstructions With Quadriceps Tendon Autograft Result in Lower Graft Rupture Rates but Similar Patient-Reported Outcomes as Compared With Hamstring Tendon Autograft: A Comparison of 875 Patients. AJSM. 2020;48(9).
9 Smith+Nephew 2021. Internal report 15011184 A.
10 Smith+Nephew 2021. Internal report 15011185 A.
11 Smith+Nephew 2020. CAP-FIX BLADE Claims Testing. Internal Report. 15009423 B.
12 Bokor DJ, Sonnabend D, Deady L, et al. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up. Muscles, Ligaments Tendons J. 2016;6(1):16-25.
13 Schlegel TF, Abrams JS, Bushnell BD, Brock JL, Ho CP. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: a prospective multicenter study. J Shoulder Elbow Surg. 2018 27(2):242-251.
14 Van Kampen C, Arnoczky S, Parks P, et al. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: a histological evaluation in sheep. Muscles Ligaments Tendons J. 2013;3(3):229-235.
15 Bokor DJ, Sonnabend DH, Deady L, et al. Healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a highly porous collagen implant: a 5-year clinical and MRI follow-up. Muscles, Ligaments Tendons J. 2019;9(3):338-347.
16 McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA, 3rd. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43(2):491-500.
17 Arnoczky SP, Bishai SK, Schofield B, et al. Histologic Evaluation of Biopsy Specimens Obtained After Rotator Cuff Repair Augmented With a Highly Porous Collagen Implant. Arthroscopy. 2017;33(2):278-283
About Smith+Nephew
Smith+Nephew is a portfolio medical technology company focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 19,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global franchises of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of $5.2 billion in 2022. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on Twitter, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as “aim”, “plan”, “intend”, “anticipate”, “well-placed”, “believe”, “estimate”, “expect”, “target”, “consider” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of COVID-19, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of COVID-19; economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers (including, without limitation, as a result of COVID-19); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of COVID-19); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew’s most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew’s expectations.
™ Trademark of Smith+Nephew. Certain marks registered US Patent and Trademark Office.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/smithnephew-to-highlight-procedural-innovations-in-sports-medicine-during-aaos-2023-annual-meeting-301764101.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/smithnephew-to-highlight-procedural-innovations-in-sports-medicine-during-aaos-2023-annual-meeting-301764101.html
SOURCE Smith & Nephew plc
Company Codes: NYSE:SNN, LSE:SN