Stoke’s TANGO antisense oligonucleotides showed dose-dependent reductions in non-productive mRNA and increases in both productive mRNA and protein levels from genes of diverse size, type and function
Stoke’s TANGO antisense oligonucleotides showed dose-dependent reductions in non-productive mRNA and increases in both productive mRNA and protein levels from genes of diverse size, type and function
BEDFORD, Mass.--(BUSINESS WIRE)-- Stoke Therapeutics, Inc. (Nasdaq: STOK), a biotechnology company pioneering a new way to treat the underlying cause of severe genetic diseases, today announced the publication of data in the journal Nature Communications that support the company’s proprietary approach to precisely upregulate protein expression using TANGO (Targeted Augmentation of Nuclear Gene Output) antisense oligonucleotides (ASOs).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200709005385/en/
TANGO (Targeted Augmentation of Nuclear Gene Output)
“Stoke was founded on the idea that we could use unique insights in RNA biology to do something that has never been done before,” said Isabel Aznarez, Ph.D., Co-Founder and Vice President, Head of Biology of Stoke Therapeutics and the corresponding author on the paper. “Rather than address genetic diseases by replacing, repairing or editing faulty genes, we set out to increase – or stoke – protein output from healthy genes. These data show that we can increase full-length, fully functional protein expression from a variety of healthy genes, which supports our hypothesis and may lead to a new way of treating severe genetic diseases.”
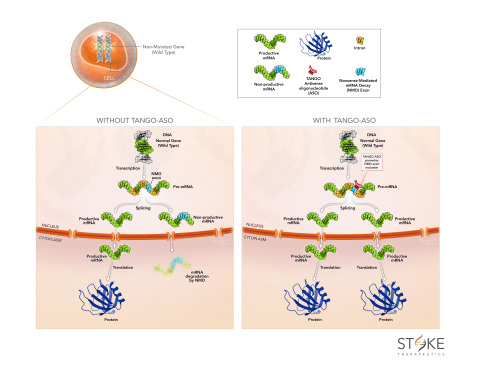
To evaluate the approach broadly, Stoke selected four gene targets that vary in type and abundance of non-productive splicing events, gene size and protein function: PCCA (propionic acidemia); SYNGAP1 (autosomal dominant mental retardation 5); CD274 (autoimmune diseases, including uveitis); and SCN1A (Dravet syndrome). Stoke designed TANGO ASOs to target the non-productive splicing events in these genes and their activity was evaluated. Dose-dependent reductions of non-productive mRNA were observed to lead to increases in both productive mRNA and protein levels for each of the target genes.
More than 10,000 genetic diseases are caused by mutations in a single gene, however, current therapeutic approaches address as few as 5% of these diseases. In the experiments published today, a proprietary bioinformatics analysis of RNA sequencing datasets was used to identify a variety of non-productive alternative-splicing events that lead to mRNA degradation and limit protein production. Stoke found 7,757 unique genes that contained at least one non-productive event, of which 16% (1,246) were associated with causing a specific disease.
A link to the publication, “Antisense oligonucleotide modulation of non-productive alternative splicing upregulates gene expression,” can be found here: https://www.nature.com/articles/s41467-020-17093-9
Pre-mRNA Splicing and TANGO
Human cells naturally regulate protein production to maintain health. Pre-mRNA splicing, including alternative splicing, is an important mechanism used to regulate how much protein and which protein variant is produced. During splicing, introns are removed and exons are joined together to generate the mRNA template that carries the code to synthesize proteins. More than one third of alternative splicing events in mammals do not produce functional proteins and lead to mRNA degradation through nonsense-mediated mRNA decay (NMD). TANGO ASOs act at the pre-mRNA level and prevent non-productive alternative splicing so that the body produces more protein-coding mRNA and thus more protein. This approach is particularly applicable to diseases that are caused by insufficient protein production.
About Stoke Therapeutics
Stoke Therapeutics, Inc. (Nasdaq: STOK), is a biotechnology company pioneering a new way to treat the underlying causes of severe genetic diseases by precisely upregulating protein expression to restore target proteins to near normal levels. Stoke aims to develop the first precision medicine platform to target the underlying cause of a broad spectrum of genetic diseases in which the patient has one healthy copy of a gene and one mutated copy that fails to produce a protein essential to health. These diseases, in which loss of approximately 50% of normal protein expression causes disease, are called autosomal dominant haploinsufficiencies. The company’s lead investigational new medicine is STK-001, a proprietary antisense oligonucleotide (ASO) that has the potential to be the first disease-modifying therapy to address the genetic cause of Dravet syndrome, a severe and progressive genetic epilepsy. Stoke is headquartered in Bedford, Massachusetts with offices in Cambridge, Massachusetts. For more information, visit https://www.stoketherapeutics.com/ or follow the company on Twitter at @StokeTx.
Forward-Looking Statements
This press release contains “forward-looking” statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: our expectations about Company’s proprietary approach to precisely upregulate protein expression using TANGO ASOs and the potential benefits thereof. These forward-looking statements may be accompanied by such words as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “might,” “plan,” “potential,” “possible,” “will,” “would,” and other words and terms of similar meaning. These forward-looking statements involve risks and uncertainties, as well as assumptions, which, if they do not fully materialize or prove incorrect, could cause our results to differ materially from those expressed or implied by such forward-looking statements. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including: risks related to the direct and indirect impact of COVID-19; our ability to develop, obtain regulatory approval for and commercialize current and future product candidates; the timing and results of preclinical studies and clinical trials; the risk that positive results in a clinical trial may not be replicated in subsequent trials or success in early stage clinical trials may not be predictive of results in later stage clinical trials; risks associated with clinical trials, including our ability to adequately manage clinical activities, unexpected concerns that may arise from additional data or analysis obtained during clinical trials; the risk that regulatory authorities may require additional information or further studies, or may fail to approve or may delay approval of our drug candidates; risks related to the occurrence of adverse safety events; risks related to failure to protect and enforce our intellectual property, and other proprietary rights; risks related to failure to successfully execute or realize the anticipated benefits of our strategic and growth initiatives; risks relating to technology failures or breaches; our dependence on collaborators and other third parties for the development, regulatory approval, and commercialization of products and other aspects of our business, which are outside of our full control; risks associated with current and potential delays, work stoppages, or supply chain disruptions caused by the coronavirus pandemic; risks associated with current and potential future healthcare reforms; risks relating to attracting and retaining key personnel; failure to comply with legal and regulatory requirements; risks relating to access to capital and credit markets; environmental risks; risks relating to the use of social media for our business; and the other risks and uncertainties that are described in the Risk Factors section of our most recent annual or quarterly report and in other reports we have filed with the U.S. Securities and Exchange Commission. These statements are based on our current beliefs and expectations and speak only as of the date of this press release. We do not undertake any obligation to publicly update any forward-looking statements.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200709005385/en/
Stoke Media & Investor Contact:
Dawn Kalmar
Vice President, Head of Corporate Affairs
dkalmar@stoketherapeutics.com
781-303-8302
Source: Stoke Therapeutics, Inc.
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20200709005385/en