Tandem Diabetes Care, Inc. announced the United States commercial launch of its new Tandem Mobi, the world’s smallest, durable automated insulin delivery system for people living with diabetes.* The company has begun taking orders and shipping to eligible customers in the United States.
SAN DIEGO--(BUSINESS WIRE)-- Tandem Diabetes Care, Inc. (NASDAQ: TNDM), a leading insulin delivery and diabetes technology company, today announced the United States commercial launch of its new Tandem Mobi, the world’s smallest, durable automated insulin delivery system for people living with diabetes.* The company has begun taking orders and shipping to eligible customers in the United States.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240213214991/en/

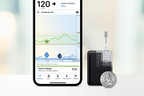
Tandem Mobi, the world’s smallest, durable insulin delivery system, now shipping in the United States. (Photo: Business Wire)
Tandem Mobi is a new way for people living with diabetes to experience the life-changing benefits of Control-IQ technology, the same technology that helped make Tandem the #1 recommended pump brand.1 The Control-IQ advanced hybrid closed-loop automated insulin delivery feature, cleared for use by people with type 1 diabetes age 6 and up, predicts and helps prevent high and low blood sugar, and has demonstrated improved time in range throughout the day and night in numerous controlled clinical trials and real-world studies.2-4
“This tiny, wearable pump exceeded the expectations of users from an early access program, and we are thrilled to begin offering this exciting new technology to more people in the diabetes community,” said John Sheridan, president and chief executive officer. “With this launch, we are executing on our strategy to offer a differentiated portfolio of durable insulin pumps, providing choice, along with new options in wearability.”
Key Tandem Mobi System Features:
- Impressively Small – Less than half the size of the t:slim X2 insulin pump and small enough to fit in the coin pocket of a pair of jeans
- Wearability Like Never Before – Can be worn almost anywhere, giving users greater discretion, comfort, and options for how they manage their diabetes:
- Wear it on-body with a lightweight adhesive sleeve (sold separately)
- Clip it discreetly to clothing
- Slip it into a pocket
- Infusion Set Options – Compatible with all existing Tandem-branded infusion sets manufactured by Convatec, including a new 5-inch tubing option made just for Tandem Mobi. Infusion sets allow users to temporarily disconnect from their pump for convenience and provide the flexibility of more than 30 mix-and-match infusion site and tubing length combinations.
- Tandem Mobi Mobile App – Full iOS mobile control through a user’s compatible iPhone5
- Sensor Compatibility – Compatible with the Dexcom G6 Continuous Glucose Monitoring (CGM) System. Additional CGM sensor integrations are planned, including Dexcom G7 in the second quarter of 2024, followed by integration with the Abbott FreeStyle Libre 3 sensor.
- #1 Rated1 Control-IQ Technology – Uses values from a Dexcom G6 CGM to predict glucose levels 30 minutes ahead and automatically adjust insulin, if needed, to help prevent highs and lows
- Clear 200-unit cartridge – Allows users to see their remaining insulin level
- Water-Resistant – The pump is water-resistant to a depth of 8 feet (2.4 meters) for up to 2 hours (IP28 rating)
- Flexible Pump Control – The Tandem Mobi system has a physical button that allows users to deliver a bolus of insulin without their iPhone.
- Advanced Technology – Uses modern wireless charging and is capable of remote software updates6
Accessing the Tandem Mobi System
Orders will be processed on a first come, first served basis, with initial shipments to users who meet specific eligibility requirements, including use of the Dexcom G6 CGM to access automated insulin delivery features. The Tandem Customer Care team is available now to help determine eligibility and potential timing of pump shipments for customers. People living with diabetes interested in the Tandem Mobi system can start the order process at: www.tandemdiabetes.com/mobi.
About Tandem Diabetes Care
Tandem Diabetes Care, a global insulin delivery and diabetes technology company, manufactures and sells advanced automated insulin delivery systems that reduce the burden of diabetes management, while creating new possibilities for patients, their loved ones, and healthcare providers. The Company’s pump portfolio features the Tandem Mobi system and the t:slim X2 insulin pump, both of which feature Control-IQ advanced hybrid closed-loop technology. Tandem Diabetes Care is based in San Diego, California. For more information, visit tandemdiabetes.com.
Follow Tandem Diabetes Care on X @tandemdiabetes; use #tslimX2 #TandemMobi and #TandemDiabetes.
Follow Tandem Diabetes Care on Facebook at www.facebook.com/TandemDiabetes.
Follow Tandem Diabetes Care on LinkedIn at https://www.linkedin.com/company/tandemdiabetes.
© 2024 Tandem Diabetes Care, Inc. All rights reserved. Tandem Diabetes Care, the Tandem logo, Control-IQ, t:slim X2, and Tandem Mobi are either registered trademarks or trademarks of Tandem Diabetes Care, Inc. in the United States and/or other countries. Dexcom, Dexcom G6, Dexcom G7, and any related logos and design marks are either registered trademarks or trademarks of Dexcom, Inc. in the United States and/or other countries. FreeStyle, Libre, and related brand marks are marks of Abbott and used with permission. iPhone is a trademark of Apple Inc., registered in the U.S. and other countries. All other third-party marks are the property of their respective owners.
Responsible Use of Control-IQ technology
Control-IQ technology does not prevent all highs and lows. Users must still bolus for meals and actively manage their diabetes. Visit tandemdiabetes.com/responsible-use for additional important safety information.
*As of Feb., 2024. Data on file, Tandem Diabetes Care.
1. dQ&A US Diabetes Connections Patient Panel Report; Net Promoter Score; Q1 2019-Q3 2023: P.49.
2. All published clinical trials and real-world studies of Control-IQ technology to date are based on use of Dexcom G6 CGM with the Tandem t:slim X2 insulin pump.
3. Breton MD, Kovatchev BP. One year real-world use of the Control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021;23(9):601-608. Doi: 10.1089/dia.2021.0097.
4. Beck RW, Kanapka LG, Breton MD, et al. A Meta-Analysis of Randomized Trial Outcomes for the t:slim X2 Insulin Pump with Control-IQ Technology in Youth and Adults from Age 2 to 72. Diabetes Technol Ther. 2023 May;25(5):329-342. doi: 10.1089/dia.2022.0558.
5. The mobile app requires a compatible iPhone model and operating system (sold separately). Only available to pump users who reside in the United States.
6. Future updates for all or some of Tandem’s products may not be developed and may not be offered everywhere and would be subject to applicable regulatory approvals. Software updates are only available to customers who are in warranty at the time they update their pump. Additional training may be required to access certain software updates. Charges may apply. Tandem may discontinue select software and features over time at its discretion.
Important Safety Information
RX ONLY. Indications for Use: Tandem Mobi system: The Tandem Mobi insulin pump with interoperable technology (the pump) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient, home use and requires a prescription. The pump is indicated for use in individuals 6 years of age and greater. Control-IQ technology: Control-IQ technology is intended for use with compatible integrated continuous glucose monitors (iCGM, sold separately) and alternate controller enabled (ACE) pumps to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons 6 years of age and greater. Control-IQ technology is intended for single patient use. Control-IQ technology is indicated for use with NovoLog or Humalog U-100 insulin.
WARNING: Control-IQ technology should not be used by anyone under the age of 6 years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds.
Control-IQ technology is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Do not use Control-IQ technology if using hydroxyurea. Users of a Tandem insulin pump and Control-IQ technology must use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders. The Tandem pump and the CGM transmitter and sensor must be removed before MRI, CT, or diathermy treatment. Visit tandemdiabetes.com/safetyinfo for additional important safety information.
Forward-looking Statements
This press release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements relate to, among other things, the anticipated integrations of the Tandem Mobi insulin pump with the Dexcom G7 and Abbott Freestyle Libre 3 CGM sensors. These statements are subject to numerous risks and uncertainties, including the risks associated with the research and development process generally, such as the design, testing, and validation in compliance with the applicable regulatory and legal requirements in the markets that we serve as well as our ability to market, scale, and maintain the systems, personnel, and infrastructure necessary to support these integrations. These and other risks are identified and described in greater detail under the “Risk Factors” heading of our most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and other documents filed with the Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this release. Actual results could differ materially from those anticipated or projected in the forward-looking statements. Tandem undertakes no obligation to update or review any forward-looking statement in this press release because of new information, future events, or other factors.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240213214991/en/
Media Contact:
858-366-6900
media@tandemdiabetes.com
Investor Contact:
858-366-6900
IR@tandemdiabetes.com
Source: Tandem Diabetes Care, Inc.
Tandem Mobi, the world’s smallest, durable insulin delivery system, now shipping in the United States. (Photo: Business Wire)
Tandem Mobi, the world’s smallest, durable insulin delivery system, now shipping in the United States. (Photo: Business Wire)
Tandem Mobi, the world’s smallest, durable insulin delivery system, now shipping in the United States. (Adhesive sleeve sold separately.) (Photo: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20240213214991/en