Theralase Technologies Inc. is providing an update regarding its Phase II Non-Muscle Invasive Bladder Cancer (" NMIBC ") clinical study (" Study II ").
TORONTO, ON / ACCESSWIRE / January 15, 2024 / Theralase Technologies Inc. (" Theralase® " or the " Company ") ( TSXV:TLT )( OTCQB:TLTFF ), a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation activated Photo Dynamic Compounds (" PDCs ") for the safe and effective destruction of various cancers, bacteria and viruses is providing an update regarding its Phase II Non-Muscle Invasive Bladder Cancer (" NMIBC ") clinical study (" Study II ").
To date, Study II has provided the primary study treatment for 63 patients.
In 2016, Kamat et al. stated in the Journal of Clinical Oncology that the International Bladder Cancer Group (" IBCG ") recommended that, " Single-arm designs may be relevant for the BCG-unresponsive population. Here, a clinically meaningful initial complete response rate (for carcinoma in situ) or recurrence-free rate (for papillary tumors) of at least 50% at 6 months, 30% at 12 months and 25% at 18 months is recommended. " [1]
Based on the 63 patients treated to date, the interim clinical data for Study II is presented below:
|
Assessment |
Primary Objective Performance |
Secondary Objective Performance |
Tertiary Objective Performance |
|||
|
# |
% |
# |
% |
# |
% |
|
|
Complete Response ("CR") |
38 |
64% |
17 |
37% |
59 |
100% |
|
Indeterminate Response ("IR") |
6 |
10% |
2 |
4% |
0 |
0% |
|
Total Response (CR and IR) |
44 |
75% |
19 |
41% |
59 |
100% |
|
Evaluable Patients |
59 |
46 |
59 |
|||
|
Assessment |
Patient Assessment Visit |
|||||||||
|
90 Days |
180 Days |
270 Days |
360 Days |
450 Days |
||||||
|
# |
% |
# |
% |
# |
% |
# |
% |
# |
% |
|
|
Complete Response ("CR") |
33 |
56% |
31 |
54% |
23 |
43% |
20 |
38% |
17 |
37% |
|
Indeterminate Response ("IR") |
4 |
7% |
10 |
18% |
7 |
13% |
3 |
6% |
2 |
4% |
|
Total Response (CR and IR) |
37 |
63% |
41 |
72% |
30 |
56% |
23 |
44% |
19 |
41% |
|
Evaluable Patients |
59 |
57 |
54 |
52 |
46 |
|||||
The Study II interim clinical data demonstrates a Complete Response (" CR ") of 54% at 6 months, 38% at 12 months and 37% at 15 months, which exceeds the IBCG guidelines.
In addition, the Study II interim clinical data demonstrates that at the 90 Day Assessment Visit, 56% of Evaluable Patients achieved a CR and 63% achieved a Total Response (CR + IR), while at 450 days, 37% achieved a CR and 41% achieved a TR.
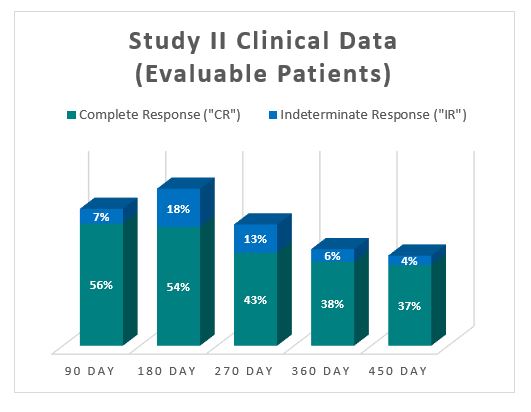
For evaluable patients in Study II, who received the optimized Study II Treatment (Post August 1, 2020), the interim clinical data is presented below:
|
Assessment |
Patient Assessment Visit (Optimized Treatment - Post August 1, 2020) |
|||||||||
|
90 Day |
180 Day |
270 Day |
360 Day |
450 Day |
||||||
|
# |
% |
# |
% |
# |
% |
# |
% |
# |
% |
|
|
Complete Response ("CR") |
29 |
62% |
28 |
60% |
20 |
45% |
17 |
40% |
14 |
39% |
|
Indeterminate Response ("IR") |
3 |
6% |
9 |
19% |
6 |
14% |
3 |
7% |
2 |
6% |
|
Total Response (CR and IR) |
32 |
68% |
37 |
79% |
26 |
59% |
20 |
48% |
16 |
44% |
The interim clinical data for patients who received the optimized Study II Treatment demonstrates that at the 90 Day Assessment Visit, 62% of Evaluable Patients achieved a CR and 68% achieved a Total Response (CR + IR), while at 450 days, 39% achieved a CR and 44% achieved a TR.
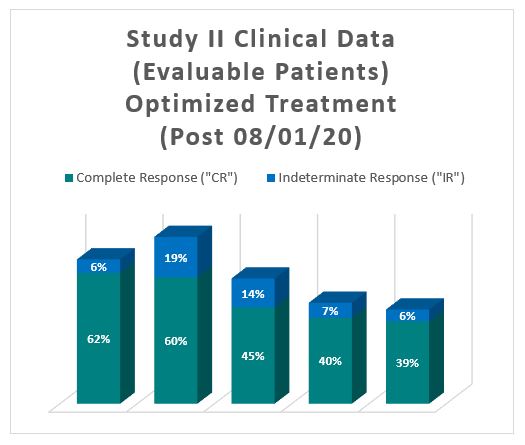
Notes:
- Indeterminate Response (" IR ") is defined as negative cystoscopy (no evidence of Urothelial Cell Carcinoma (" UCC ") in the bladder) and positive urine cytology (detection of cancer in the urine, without a negative confirmatory bladder biopsy, suggesting UCC in the renal system other than the bladder)
- For patients to be included in the statistical clinical analysis they must be enrolled in Study II, provided the primary Study II Treatment and evaluated by a PI at the 90 day assessment visit (cystoscopy and urine cytology)
- One patient passed away prior to their 90 day assessment and is therefore not included in the statistical analysis; therefore, there are 63 patients in the statistical analysis.
- Evaluable Patients are defined as patients who have been evaluated by a PI and thus the statistical analysis excludes a patient's clinical data at specific assessment days, if that clinical data is pending.
- Four patients have been enrolled and provided the primary Study II Treatment, but have not been evaluated at their 90 day assessment; therefore, 59 patients are considered Evaluable Patients at 90 days and 46 patients considered Evaluable Patients at 450 days.
- The data analysis presented above, should be read with caution, as the clinical data is interim in its presentation, as Study II is ongoing and new clinical data collected may or may not continue to support the current trends, with clinical data still pending.
- For patients who have been removed from Study II by the PI or have elected to discontinue from Study II their Last Observation Carried Forward (" LOCF ") has been used in this statistical analysis.
- A SAE is defined as any untoward medical occurrence that at any dose: Is serious or life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, is a congenital anomaly/birth defect or results in death.
The Swimmer's plot below is a graphical representation of the interim clinical results (n=63) graphically demonstrating a patient's response to a treatment over time. As can be seen in the plot, clinical data is still pending for patients, who have demonstrated an initial CR at 90 days and continue to demonstrate a duration of that response.
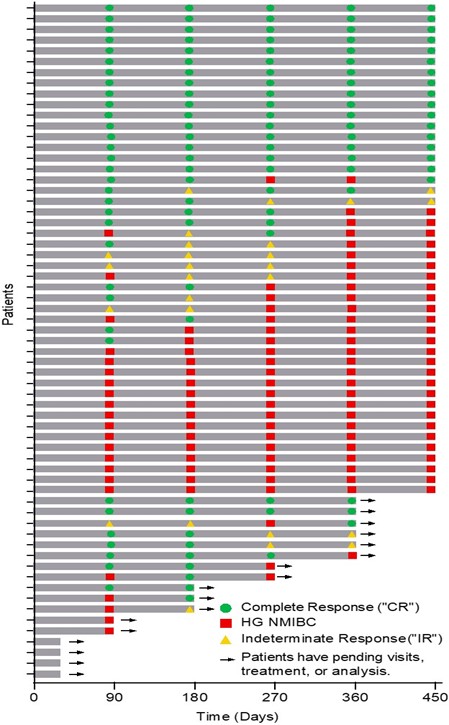
The Swimmer's Plot illustrates:
- 38 Evaluable Patients achieved CR on at least one assessment date and thus achieved the primary objective of Study II (38/59 = 64%)
- 17 Evaluable Patients achieved CR at each assessment date (with one patient under review for their 270 and 360 day response) and thus achieved the primary and secondary objectives of Study II for all patients assessed up to 450 days (17/46 = 37%).
The Kaplan-Meier (" KM ") Curve below represents the interim cumulative incidence of clinical events; including, the treatment efficacy occurring over a prespecified time in Study II.
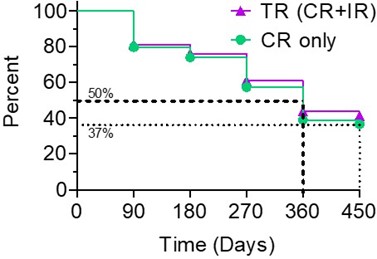
According to the interim clinical data in the KM curve:
- > 80% of patients remained in Study II after 90 days, following the initial Study II Treatment.
- For all evaluable patients, 41% of Total Response (" TR ") have a duration of response ≥ 450 days, while 37% of Complete Response (" CR ") evaluable patients have a duration of response ≥ 450 days.
- For optimized evaluable patients, 44% of TR patients have a duration of response ≥ 450 days and 39% of CR have a duration of response ≥ 450 days.
For 63 patients treated in Study II, there have been 13 Serious Adverse Events (" SAEs ") reported:
- 2 - Grade 2 (resolved within 1 and 1 days, respectively)
- 7 - Grade 3 (resolved within 1, 2, 3, 4, 4, 82 and unknown days, respectively)
- 3 - Grade 4 (resolved within 3, 6 and 8 days, respectively)
- 1 - Grade 5
Theralase® believes all SAEs reported to date are unrelated to the Study II Drug or Study II Device.
In 2020, the FDA granted Theralase® Fast Track Designation (" FTD ") for Study II. As a Fast Track designee, Theralase® has access to early and frequent communications with the FDA to discuss Theralase®'s development plans and ensure the timely collection of clinical data to support the approval process. The accelerated communication with the FDA potentially allows, the Study II Treatment, to be the first intravesical, patient-specific, light-activated, Ruthenium-based PDC for the treatment of patients diagnosed with BCG-Unresponsive NMIBC CIS, (with or without recurrent / resected papillary T a /T 1 tumours). FTD can lead to Break Through Designation (" BTD "), Accelerated Approval (" AA ") and/or Priority Review, if certain criteria are met.
In mid-2023, the Company submitted a pre-BTD submission to the FDA and based on the FDA's feedback, the Company is currently working with the Clinical Study Sites (" CSSs "), a central pathology organization, a biostatistics organization and a regulatory consulting organization to update the pre-BTD with clinical data clarifications identified by the FDA. The Company plans to resubmit the pre-BTD submission to the FDA in 1Q2024 for FDA review of these clarifications. Once the pre-BTD submission has been accepted by the FDA, the Company plans to compile a BTD submission for review by the FDA in support of the grant of a BTD approval.
Theralase® is working to complete enrollment and delivery of the primary Study II Treatment for all patients in 2024. If successful, this would allow clinical data lock in mid-2026 with a potential Health Canada and FDA approval by 2026 / 2027.
About Study II:
Study II utilizes the therapeutic dose of the patented Study II Drug (" Ruvidar TM " or " TLD-1433 ") (0.70 mg/cm 2 ) activated by the proprietary Study II Device (" TLC-3200 Medical Laser System "). Study II is focused on enrolling and treating approximately 100 BCG-Unresponsive NMIBC Carcinoma In-Situ (" CIS ") patients in up to 15 CSSs located in Canada and the United States.
About Ruvidar TM :
Ruvidar TM is a patented PDC with 12 years of published peer reviewed preclinical research and is currently under investigation in Study II.
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light activated compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains "forward-looking statements" within the meaning of applicable Canadian securities laws. Such statements include; but are not limited to statements regarding the Company's proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Forward looking statements may be identified by the use of the words " may , " should ", " will ", " anticipates ", " believes ", " plans ", " expects ", " estimate ", " potential for " and similar expressions; including, statements related to the current expectations of Company's management for future research, development and commercialization of the Company's Photo Dynamic Compounds and their drug formulations, preclinical research, clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to: adequately fund, and secure the requisite regulatory approvals to successfully complete a Phase II NMIBC clinical study in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its drug formulations, the risk that access to sufficient capital to fund the Company's operations may not be available or may not be available on terms that are commercially favorable to the Company, the risk that the Company's drug formulations may not be effective against the diseases tested in its clinical studies, the risk that the Company's fails to comply with the term of license agreements with third parties and as a result loses the right to use key intellectual property in its business, the Company's ability to protect its intellectual property, the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these forward- looking statements which are not a guarantee of future performance. There can be no assurance that forward looking statements will prove to be accurate as such forward looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the forward-looking statements.
Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements.
All forward-looking statements are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
khachey@theralase.com
[1]Kamat AM et al. J Clin Oncol. 2016; 34: 1935-1944
SOURCE: Theralase Technologies Inc.
View the original press release on accesswire.com




