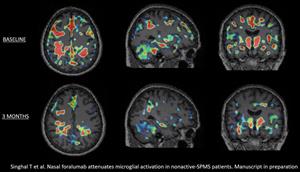
Tiziana Life Sciences Ltd. announced a reduction in microglial activation as seen in 3-month Positron Emission Tomography scans that has now been seen in a total of 5 of the 6 patients with non-active secondary-progressive multiple sclerosis treated with intranasal foralumab in its Expanded Access program.
- Results confirm positive signal previously reported in the first two Expanded Access patients who both subsequently demonstrated clinical improvements
- Phase 2a trial in na-SPMS on track to begin in Q3 2023
- KOL webinar with Howard L. Weiner, M.D., Chairman of Tiziana’s Scientific Advisory Board and Co-Director of the Ann Romney Center for Neurologic Diseases at Brigham and Women’s Hospital being held today at 12:30 PM ET (details below)
NEW YORK, June 05, 2023 (GLOBE NEWSWIRE) -- Tiziana Life Sciences Ltd. (Nasdaq: TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies via novel routes of drug delivery, today announced a reduction in microglial activation as seen in 3-month Positron Emission Tomography (PET) scans that has now been seen in a total of 5 of the 6 patients with non-active secondary-progressive multiple sclerosis (na-SPMS) treated with intranasal foralumab in its Expanded Access program. Activated microglia are believed to play a prominent role in the pathogenesis of neuroinflammatory diseases including multiple sclerosis, Alzheimer’s disease and amyotrophic lateral sclerosis (ALS).
A reduction in microglial activation is associated with lowered inflammation in the brain. Inflammation in the brain drives the disease pathology in multiple sclerosis. In SPMS, inflammation in the brain occurs in microglia, the brain’s immune cells, which drive the neurodegeneration of brain cells. During the inflammatory process associated with SPMS, microglia are involved in the destruction of myelin, the protective sheath covering of nerve fibers, and contribute to the formation of MS lesions.
Tarun Singhal, M.B.B.S., M.D., Director of PET Imaging Program in Neurologic Diseases, associate neurologist and nuclear medicine physician at Brigham and Women’s Hospital, a founding member of Mass General Brigham Healthcare System, and Assistant Professor of Neurology at Harvard Medical School, commented, “After review of the baseline and 3-month PET scans of the latest cohort of 4 Expanded Access patients, I have determined that 3 out of the 4 patients had a reduction in the microglial PET signal. An example of this can be seen in the graphic below, titled, “Figure 1”, showing the deactivation of this signal in patient EA6. When combined with my assessment of the first 2 Expanded Access patients, a total of 5 out of the 6 had a reduction in qualitative microglial PET signal, which appears to be clearly more significant than what we have identified in our test-retest assessments. I look forward to studying more patients, with full quantitation, and particularly, the next 4 patients in the Expanded Access program to see if this finding is replicated. We are preparing to submit the important results from this trial for publication.”
Figure 1.
Howard L. Weiner, M.D., Chairman of Tiziana’s Scientific Advisory Board and Co-Director of the Ann Romney Center for Neurologic Diseases at Brigham and Women’s Hospital, a founding member of Mass General Brigham Healthcare System, stated, “To see a reduction in microglial activation in 5 out of 6 na-SPMS patients in only 3 months is extraordinary. This finding is even more remarkable because all of the 6 expanded access patients’ na-SPMS had clinically regressed on ocrelizumab treatment. I am excited to follow this program to see if the improvement in the 3-month PET scans will translate to clinical improvement in the coming months.”
Dr. Tanuja Chitnis, M.D., Principal Investigator and Professor of Neurology at Harvard Medical School (HMS) and senior neurologist at Brigham and Women’s Hospital, added, “There are currently no FDA-approved treatments for na-SPMS. The reduction in microglial activation in the 3-month PET scans in 5 out of 6 patients is truly encouraging and I look forward to getting the 3-month PET scans results of the next 4 Expanded Access patients later in 2023 and to starting the Phase 2a trial this year.”
“I believe that Tiziana and Harvard are at the forefront of research in neuroinflammatory diseases with unmet need,” noted Gabriele Cerrone, Executive Chairman, Founder, and acting Chief Executive Officer of Tiziana. “Our Phase 2a multi-center, double-blinded, placebo-controlled trial in na-SPMS uses the 3-month PET scan as the primary outcome measure and our Expanded Access data from the first 6 patients give us increasing conviction in the potential for a positive outcome. We believe this trial design will provide a quick validation of our intranasal foralumab asset and will allow the company to proceed to the next clinical phase of development in na-SPMS.”
KOL Webinar information:
Time: Monday, June 5, 2023 at 12:30 PM ET
Topic: Howard Weiner, M.D., will discuss anti-inflammatory approaches in treating neurodegenerative disease and will provide an update on the ongoing intranasal foralumab Expanded Access program in na-SPMS
A live question and answer session will follow the formal presentations. To register for the event, please click: https://lifescievents.com/event/tiziana/
About Foralumab
Activated T cells play an important role in the inflammatory process. Foralumab, the only fully human anti-CD3 monoclonal antibody (mAb), binds to the T cell receptor and dampens inflammation by modulating T cell function, thereby suppressing effector features in multiple immune cell subsets. This effect has been demonstrated in patients with COVID and with multiple sclerosis, as well as in healthy normal subjects. Intranasal foralumab Phase 2 trials are expected to start in the third quarter of 2023 in patients with non-active SPMS. Immunomodulation by nasal anti-CD3 mAb represents a novel avenue for treatment of inflammatory human diseases.1
About Tiziana Life Sciences
Tiziana Life Sciences is a clinical-stage biopharmaceutical company developing breakthrough therapies using transformational drug delivery technologies to enable alternative routes of immunotherapy. Tiziana’s innovative nasal approach has the potential to provide an improvement in efficacy as well as safety and tolerability compared to intravenous (IV) delivery. Tiziana’s lead candidate, intranasal foralumab, which is the only fully human anti-CD3 mAb, has demonstrated a favorable safety profile and clinical response in patients in studies to date. Tiziana’s technology for alternative routes of immunotherapy has been patented with several applications pending and is expected to allow for broad pipeline applications.
Forward-Looking Statements
Certain statements made in this announcement are forward-looking statements. These forward-looking statements are not historical facts but rather are based on the Company’s current expectations, estimates, and projections about its industry; its beliefs; and assumptions. Words such as ‘anticipates,’ ‘expects,’ ‘intends,’ ‘plans,’ ‘believes,’ ‘seeks,’ ‘estimates,’ and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance and are subject to known and unknown risks, uncertainties, and other factors, some of which are beyond the Company’s control, are difficult to predict, and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. The Company cautions security holders and prospective security holders not to place undue reliance on these forward-looking statements, which reflect the view of the Company only as of the date of this announcement. The forward-looking statements made in this announcement relate only to events as of the date on which the statements are made. The Company will not undertake any obligation to release publicly any revisions or updates to these forward-looking statements to reflect events, circumstances, or unanticipated events occurring after the date of this announcement except as required by law or by any appropriate regulatory authority.
For further inquiries:
Tiziana Life Sciences Ltd
Paul Spencer, Business Development and Investor Relations
+44 (0) 207 495 2379
email: info@tizianalifesciences.com
Investors:
Irina Koffler
LifeSci Advisors, LLC
+1 646 970 4681
ikoffler@lifesciadvisors.com
______________________
1 https://www.pnas.org/doi/10.1073/pnas.2220272120.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/c3d42195-bf6a-4948-8422-d74ab828e620