Triastek, Inc. (“Triastek”) recently announced that the United States Food and Drug Administration (FDA) has granted permission to begin clinical studies of its Investigational New Drug (IND) 505(b)(2) application for a 3D printed drug product – T20.
|
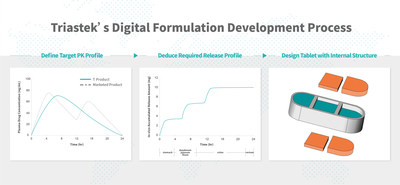
NANJING, China, April 7, 2022 /PRNewswire/ -- Triastek, Inc. ("Triastek") recently announced that the United States Food and Drug Administration (FDA) has granted permission to begin clinical studies of its Investigational New Drug (IND) 505(b)(2) application for a 3D printed drug product – T20. It is Triastek's second product receiving IND clearance from the FDA. T20 provides once daily dosing while being anticipated to maintain the same efficacy and adverse effect profile and improve adherence. The currently marketed product is given twice daily for the treatment of cardiovascular and clotting disorders and has been at or near the top of global sales for the past several years. Following the 505(b)(2) pathway, T20 is expected to provide a formulation that addresses unmet patient needs by improving adherence and therefore the opportunity for improved patient outcomes. The T20 once-daily formulation is being developed using the digital formulation development process and unique programmed drug release technology pioneered by Triastek. Using Triastek's 3D printing formulation by design (3DFbD®) method, starting with the desired extended release PK profile, the required in vivo gastrointestinal tract (GIT) dissolution-time/location profile is predicted using a physiologically-based biopharmaceutical model (PBBM) of GIT absorption to inform formulation development. From this, the internal tablet geometry can be created using Triastek's Melt Extrusion Deposition (MED®) 3D printing technology to achieve the desired release profile and resulting drug pharmacokinetics. This innovative and efficient process overcomes many of the limitations of traditional extended release product development and manufacturing. Proof of concept has already been demonstrated in animal studies using a prototype formulation of T20. Triastek is a novel 3D printing pharmaceutical technology platform company with the proprietary technologies encompassing dosage form design, digital pharmaceutical product development, and intelligent manufacturing. Using these technologies, Triastek develops its own product pipeline as well as co-developing products with multinational and Chinese pharmaceutical companies partners utilizing both the 505(b)(1) and 505(b)(2) regulatory pathways. Applying their MED® 3D printing technology, Triastek can develop novel formulations that are challenging to achieve with conventional dosage form technology to address unmet clinical needs and improve drug therapy outcomes. Focusing initially on blockbuster small molecule drugs, their development will demonstrate the utility of Triastek's novel pharmaceutical product development technology and continuous GMP manufacturing capabilities. Dr. Senping Cheng, founder and CEO of Triastek, said, "It usually takes 30 years for an emerging pharmaceutical technology to complete its journey from initial concept to marketplace. 3D printing technology as applied to the development of pharmaceuticals has been explored for over 26 years. The FDA IND clearance of T20 is a significant milestone for Triastek, and demonstrates the significant progress in 3D printing pharmaceuticals."
SOURCE Triastek, Inc. |