Xenocor, creator of the FDA cleared Saberscope, the world’s first true HD, fog-free, articulating, single-use laparoscope, announced a $10 million Series A funding led by GenHenn Venture fund.
|
Led by GenHenn Venture Fund I, Xenocor will leverage the capital to facilitate launch and rapid adoption of the 5mm articulating Saberscope SALT LAKE CITY, April 18, 2023 /PRNewswire/ -- Xenocor, creator of the FDA cleared Saberscope, the world's first true HD, fog-free, articulating, single-use laparoscope, announced a $10 million Series A funding led by GenHenn Venture Fund I with participation from Baranco Investments, Inc., Barvest Ventures, Inc. and Patel Family Investments. Xenocor will use the capital for the 2023 launch of the Saberscope and to continue executing on its robust innovation roadmap. To help accelerate the company's growth, Michael J. Hennessy Jr., GenHenn Capital will join the Xenocor board of directors. Xenocor is facilitating the evolution of the 35-year-old MIS visualization model to better serve care givers and patients
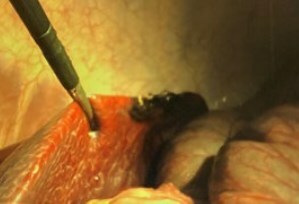
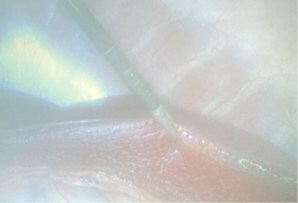
"Xenocor is laser focused on facilitating the evolution of the 35-year-old laparoscopic visualization model to something that clearly better serves care givers and patients during minimally invasive surgery," shared Charles DeCoster IV, CEO of Xenocor. "Our FDA cleared Saberscope improves visualization, dramatically diminishes workflow complexity, increases safety and reduces waste." With an addressable market of $4.5 billion in the U.S. alone, Xenocor has created the best technology on a scalable platform, hired the right veteran medical device leadership and is following the right strategic course to capture meaningful share of this dynamic market. "We are beyond thrilled to be leading the Xenocor Series A. Based on the deep insights I have gathered during my long career in healthcare leadership, I believe Xenocor will have a transformational impact on minimally invasive surgery," shared Michael J. Hennessy Jr., GenHenn managing partner. "Their unique technology coupled with their veteran leadership will change the future of laparoscopy forever." The Time for Single-Use is Now The current reusable laparoscope model has been in place for 35 years and is the only option for minimally invasive surgeons. On a typical day in a hospital, before the surgeon enters the room, the staff has already brought in the equipment, warmed the scopes in a saline bath and put on a special light cord setting to avoid starting an OR fire. When the surgeon enters, they have to white balance the scope and 62.5% of the time the equipment is still not ready, and the surgeon needs to spend time troubleshooting(1). During the surgery the scope frequently fogs, it is difficult to see through smoke and steam and almost impossible to see around critical anatomy. Once the procedure is complete, the devices are wiped down and transported to sterile processing (usually not just around the corner from the OR). At sterile processing, staff are in incredibly short supply nationwide, have to thoroughly clean the scopes and properly route broken equipment through a complex repair and loaner process. The scopes that are deemed to be in working order must then be transported to the right place at the right time, which is no easy feat. The Saberscope addresses every unmet need described above. When the staff get into the room, they open the Saberscope, plug it in and go. The Saberscope eliminates fog, sees better through smoke and steam and can articulate 90 degrees in any direction with a simple wrist movement, allowing the surgeon full visibility to the anatomy they need at any point during the procedure. Lastly, the scope is placed in a Xenocor recycle bin and a new one can be easily pulled off the shelf. It just makes sense. The below images are at the same time in the same anatomy and they show the stark difference between the Saberscope and the current reusable technology. Xenocor is Ramping Capabilities Due to the significant positive feedback received from across the laparoscopic community, Xenocor is investing in industry leading sales talent to support the long list of facilities where surgeon champions are ushering the Saberscope through the procurement process. A number of large academic systems have already issued purchase orders. Xenocor has brought in additional manufacturing leadership to drive meaningful scale. Given the significant demand, new healthcare systems will be brought on in sync with the manufacturing plan, so no customer will have to experience disruptions in service. Xenocor is building the operational capabilities to provide top of the line support to its incredible surgical champions and administrative allies. Xenocor's culture puts the customer at the center, so investment into customer experience is critical at this stage. About Xenocor Xenocor is a privately held company that designs, develops, and commercializes medical devices including the Saberscope system. The Saberscope system is FDA cleared and is intended to be used in diagnostic and therapeutic procedures for endoscopy and endoscopic surgery within the thoracic and peritoneal cavities including the female reproductive organs. Live procedure footage: https://vimeo.com/816722218 Xenocor contact: (844) 936-6267; info@xenocor.com. About GenHenn Capital GenHenn Capital is a family office venture fund established to invest in technology focused life sciences companies at all stages of their life cycle.
SOURCE Xenocor, Inc. |