Xstim, Inc., a pioneering developer and manufacturer of cutting-edge bone growth stimulation systems, is thrilled to announce its recent Premarket Application approval from the U.S. Food and Drug Administration for Xstim™ Spine Fusion Stimulator.
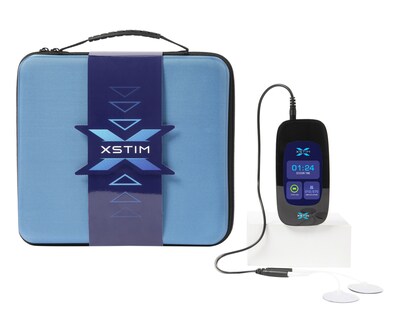
IRVING, Texas, April 16, 2024 /PRNewswire/ -- Xstim, Inc., a pioneering developer and manufacturer of cutting-edge bone growth stimulation systems, is thrilled to announce its recent Premarket Application (PMA) approval from the U.S. Food and Drug Administration (FDA) for Xstim™ Spine Fusion Stimulator. Engineered with patient comfort and convenience in mind, the Xstim™ Spine Fusion Stimulator represents a significant milestone in the company's product lineup of bone growth stimulators. This capacitively coupled device emits a low-energy signal clinically proven to promote bone healing after spinal fusion surgery1. Commercial availability of the Xstim device is slated for the second quarter of this year, with a targeted and phased launch plan in place to ensure widespread accessibility.
The Xstim device has been meticulously designed to prioritize patient ease of use and wearability, offering a noninvasive alternative for indicated cases. With its intuitive interface and sleek design, featuring a large onboard color display, the Xstim device streamlines patient therapy management.
Dr. Adam Bruggerman, MD, commented on the significance of Xstim, stating, "Bone healing following spinal fusion surgery is paramount to successful patient outcomes. The availability of non-invasive bone growth stimulation options like Xstim provides my patients with an invaluable advantage. Its sleek design ensures simple therapy management without the hassle of downloading and managing extra apps."
Jeremy Perkins, Chief Executive Officer of Xstim, Inc., expressed the company's commitment to enhancing spinal fusion rehabilitation standards, saying, "Xstim, Inc. is dedicated to empowering patients and surgeons to elevate the quality of care in spinal fusion rehabilitation. The introduction of the Xstim Spine Fusion Stimulator represents the beginning of our robust pipeline of bone growth stimulation innovations. We are poised to collaborate with healthcare providers and distribution partners to enhance bone fusion outcomes and elevate patient satisfaction. This FDA approval underscores our leadership in and dedication to the bone growth stimulation market."
About Xstim, Inc.
Xstim, Inc. is a progressive medical technology company dedicated to transforming bone growth stimulation solutions. Centered on innovation, patient well-being, and clinical effectiveness, its mission is to redefine the care benchmark in spinal fusion rehabilitation and beyond.
For prescribing information and more about Xstim, Inc., please visit www.xstimmed.com.
For Media Inquiries:
Tony Spyropouolos
Tony@xstimmed.com
References:
- Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine. 1999;24(13):1349-1356.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/xstim-inc-receives-fda-approval-for-xstim-spine-fusion-stimulator-302116227.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/xstim-inc-receives-fda-approval-for-xstim-spine-fusion-stimulator-302116227.html
SOURCE Xstim, Inc.