Before cell and gene therapy can truly become mainstream medicine, the challenges of allogeneic rejection and targeted delivery must be solved. Sana Biotechnology is on the path to do that.
A Sana scientist works in the lab/Photo courtesy of Sana Biotechnology
One of the most important transformations of 21st-century medicine will be the ability to reengineer cells as medicines. Before cell and gene therapy can truly become mainstream medicine, the challenges of allogeneic rejection and targeted delivery must be solved. Sana Biotechnology is on the path to do that, with notable strides that may, just possibly, change medicine as we know it.
“Our aspirations are to be able to repair or control genes and gene expression of every cell type in the body that’s broken, and to transplant or replace what’s missing or too far damaged,” Steve Harr, M.D., Sana’s president and CEO, told BioSpace.
The scope of that mission is huge. “The breadth of our aspirations set Sana apart from most biotech companies,” he said. And, Sana is approaching the challenge like any other great endeavor: one step at a time. In this case, that means with ex vivo and in vivo cell engineering platforms.
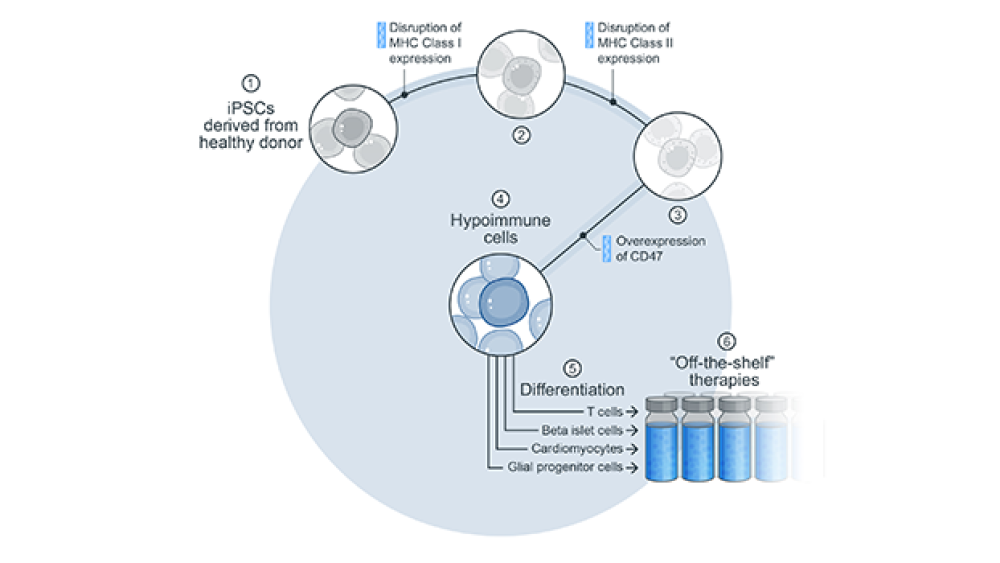
Sana’s ex vivo hypoimmune platform (HIP) is designed to prevent allogeneic rejection, in which the immune system recognizes modified cells as foreign and attacks them. “This is perhaps the greatest issue that has held cell therapy back from reaching its potential for patients,” Harr said.
The hypoimmune platform appears to solve that hurdle, he said. “We have shown in preclinical models that we have the potential to prevent allogeneic rejection.”
As Sonja Schrepfer, M.D., Ph.D., head of Hypoimmune Platform, elaborated, “The human leukocyte antigens (HLA) on a cell’s surface are like fingerprints for proteins on the cell. They tell the immune system what is inside your cell. We recognized HLA as a major hurdle (in overcoming rejection) and so prevented expression of the HLA. Now the cell’s ‘fingerprint’ is missing.”
“Of course, viruses figured out long ago that removing HLA can hide cells, so the immune system evolved to be smarter,” she continued. “NK cells and macrophages recognize cells without HLA (“missing self”) and attack them. To protect those cells from this attack, we overexpress CD47, which basically is a ‘don’t attack me’ molecule. It protects these cells from being recognized by NK cells and macrophages, allowing them to survive.”
These cellular modifications can be performed on pluripotent stem cells, which can then be differentiated into essentially any hypoimmune cell, and also on allogeneic donor cells. “We intend to derive hypoimmune pancreatic islet cells to treat type 1 diabetes, for example. Our goal is to provide off-the-shelf cells to anyone, anywhere, at any time that can function without requiring the patient to take immunosuppressive medications,” Schrepfer said. Sana also is working to develop T cells, glial progenitor cells and cardiomyocytes as allogeneic therapies.
In preclinical models, “We have seen that without immunosuppression, the HIP-edited induced pluripotent stem cells (iPSCs) survived for many weeks without activating the immune system or being rejected,” Schrepfer said. “That was very exciting!” The company is planning to file its first IND to test this system in patients as early as this year.
Targeted gene delivery is the second big challenge Sana is addressing. “With antiviral vectors (AAVs), you can’t control where the vectors go and, because the cellular DNA is not changed, they have less utility in dividing cells. Lipid nanoparticles (LNPs), another popular delivery vehicle, at this time mainly go to the liver and deliver only RNA and proteins, not DNA,” Harr said.
Sana’s fusogen platform has the potential to overcome those limitations with in vivo cell and gene modification. “You can make just about any modification to genes and gene expression in a petri dish,” Harr pointed out, “but it is very difficult to deliver the gene modification payload in vivo. Fusogens offer a unique delivery capability for gene editing and gene modification machinery. We have a number of programs in preclinical development, including four that modify T cells for multiple oncology indications, one that modifies hepatocytes to address liver-related disorders and one that modifies hematopoietic stem cells to address hemoglobinopathies.”
Sana’s science has the potential to disrupt the cell and gene therapy sector of the industry, but its effects on the practice of medicine are still at least several years away. As Harr said, “Our key competitive advantage, as a smaller company, needs to be decision-making, which is possible because of greater focus and having the right people.”
Harr defines “the right people” as program heads who are global leaders in their fields. “That’s really important,” Harr explained, “because this isn’t as easy as normal drug development (which, itself, isn’t easy).” For example, Richard Mulligan, Ph.D., head of SanaX, is instrumental in Sana’s work to deliver genomic material. SanaX is a distinct research arm within Sana focused around long-term, disruptive improvements in cell and gene therapy. Mulligan was among the first to discover how to insert genes into cells in the 1980s. Terry Fry, M.D., SVP and head of T Cell Therapeutics, is a renowned expert in chimeric antigen receptor T cell (CAR T) therapies and was critical in the development of a number of CAR T cell therapies to date, including Yescarta. Chuck Murry, M.D., Ph.D., SVP and head of cardiometabolic cell therapy, pioneered the use of human pluripotent stem cells for heart regeneration.
The executive leaders also bring a wealth of experience. Some, including Harr, joined Sana after senior positions at Juno Therapeutics. Others honed their skills at Genentech, Amazon, Amgen, Sangamo and the U.S. Food and Drug Administration, amongst others.
Sana’s staff of approximately 400 is divided among its facilities in South San Francisco, Seattle and Cambridge, Massachusetts.
The company is expanding, so there are opportunities throughout Sana both geographically and in terms of business and technical specialties. Harr said one hiring priority is in the area of process and analytical development for manufacturing. Another area includes hiring experts in analytic genomics and computational biology to enable in-depth interrogation of the genome, “both when you’re inserting things into the cells and as stem cells divide and differentiate,” he added.
“Our goal is to make important medicines that matter for patients, and these roles are essential in doing that.” That means the Sana team must also be flexible and resilient.
“The world is constantly changing, and we live on the tip of the innovation spear,” Harr said. “We need people who are intellectually curious and who have grit. Being a pioneer is hard. We will have great days, and we also will have setbacks. We need people who are resilient and who can collaborate effectively on teams. The things we are doing are complex enough that none of us have all the answers. It always takes some of us.”
Harr said Sana is committed to “ensuring we do all we can to increase individuals’ chances to succeed and rewarding them fairly. Biotech tries to help people from all over the world. To do that, we need an environment where people can thrive.”
Harr continued, “Inclusion is the soil from which all great cultures grow. To truly feel included, you need diversity. It helps to know that there are people ‘just like me’ who’ve succeeded here. Succeeding is easier when you have that example.”
With that philosophy, Sana’s Inclusion, Diversity and Equity program is robust. “The measure of a great culture is whether it helps you succeed or holds you back. Our measure is to help you be your best self, your true self and to accept people for who they are. This is an exciting time to be at Sana,” Harr said.
Sana is transforming from a research company to a research and development company. The movement into development is an important next step, Harr said. “It’s the chance to prove things work.”
Sana is working towards developing important cell and gene therapies for patients. As Harr said, “This is a dynamic company with complex science. We have chosen to be audacious.”
Featured Jobs on BioSpace