Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN; the “Company” or “Biohaven”) today announced the acquisition of the remaining 58% interest of Kleo Pharmaceuticals, Inc. (“Kleo”) that it did not previously own and the execution of an exclusive license agreement with Yale University for a novel extracellular degrader technology licensed from the Spiegel Lab
|
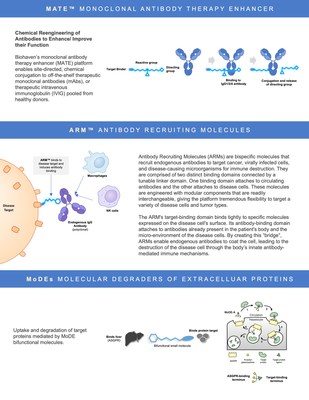
NEW HAVEN, Conn., Jan. 7, 2021 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN; the "Company" or "Biohaven") today announced the acquisition of the remaining 58% interest of Kleo Pharmaceuticals, Inc. ("Kleo") that it did not previously own and the execution of an exclusive license agreement with Yale University for a novel extracellular degrader technology licensed from the Spiegel Lab. In connection with these two transactions, Biohaven assumed Kleo's laboratory facilities located in Science Park in New Haven, Connecticut and formed Biohaven Labs to serve as the integrated chemistry and discovery research arm of Biohaven. Biohaven Labs will continue several existing Kleo discovery partnerships, including with the Bill and Melinda Gates Foundation for the development of a Hyperimmune Globulin Mimic (HGM) for COVID-19 and PeptiDream for the development of immuno-oncology therapeutics. Vlad Coric, M.D., Chief Executive Officer of Biohaven commented, "Inherent to the value creation of biopharmaceutical companies is the ability to continue to develop innovative technology platforms to deliver future treatments to patients. With these two transactions, Biohaven is excited to formally launch Biohaven Labs at Science Park in New Haven, Connecticut. Biohaven Labs will combine two cutting-edge platform technologies from Yale University in immune modulation (MATE and ARM) plus their extracellular target degrader technology with our existing small molecule discovery programs. We have a world class development team that has demonstrated the ability to advance novel treatments to the clinic and will harness the full potential of these novel technology platforms to create value for patients and investors." Kleo Pharmaceuticals Acquisition Kleo Pharmaceuticals is a privately held biotechnology company developing next-generation, bispecific compounds designed to emulate or enhance the activity of biologics based on the groundbreaking research of its scientific founder Dr. David Spiegel at Yale University. Its proprietary technology platforms are modular in design and enable rapid generation of novel immunotherapies that can be optimized against specified biological targets and combined with existing cell- or antibody-based therapies. These include Antibody Recruiting Molecules (ARMs) and Monoclonal Antibody Therapy Enhancers (MATEs). David A. Spiegel, MD, PhD commented, "I am delighted to continue working with Biohaven on the ARM and MATE platforms, and also to expand our collaboration to include Molecular Degraders of Extracellular Proteins (MoDEs). Vlad and the Biohaven team are a visionary group with the ability to bring all of these technologies into the clinic. Their demonstrated skill in clinical development dovetails perfectly with my laboratory's expertise in developing new mechanistic paradigms for therapeutic function. It is truly humbling to be a part of an enterprise with such far-reaching potential to deliver therapies for treating conditions in the oncology, autoimmunity, neuroscience realms and beyond. Plus our continuing collaborative relationship with Patrick Reid and the PeptiDream team will be an important catalyst for success." Biohaven's acquisition of Kleo was completed in a stock deal to acquire all shares of the company in the merger. In the acquisition of Kleo, for each Kleo share owned, Kleo stockholders have the right to receive, (i) approximately 0.007 of a common share of the Company, (ii) one contingent value right, representing the right to receive $1.00 in cash if certain specified Kleo biopharmaceutical products or product candidates receive the approval of the U.S. Food and Drug Administration prior to the expiration of 30 months following the effective time of the merger, and (iii) certain other amounts to extent released from escrows established to provide for indemnification claims. The agreement was unanimously approved by Biohaven's Board of Directors and was approved by Kleo's Board of Directors based upon the unanimous recommendation of a special committee of independent directors of Kleo. The merger values Kleo, including cash on hand, at approximately $20 million, exclusive of the value of the contingent value rights. With this transaction, Biohaven welcomes the Kleo chemistry and discovery staff fully into its world class clinical development operations, commercial infrastructure, and broad capital base to advance the company's product development and more effectively reach patients in need. Biohaven Labs will assume full control of the approximately 10,000 square feet of the recently established Kleo chemistry and discovery facilities at Science Park in New Haven, Connecticut. Biohaven Labs will continue its collaboration with PeptiDream and plans on expanding its partnership. Patrick C. Reid, PhD, Chief Executive Officer of PeptiDream commented, "We greatly look forward to working with the Biohaven clinical development and Biohaven Labs teams on advancing the next generation of therapeutics, with the ultimate goal of bringing these exciting therapies to commercialization and improving the lives of patients worldwide." Yale University License Agreement for Spiegel Lab Degrader Technology Biohaven has entered into a worldwide, exclusive license agreement for the development and commercialization of a novel Molecular Degrader of Extracellular Protein (MoDEs) platform based on ground-breaking research conducted in the laboratory of Professor David Spiegel at Yale University. Under the license agreement, Biohaven acquired exclusive, worldwide rights to Yale's intellectual property directed to its MoDEs platform. The platform pertains to the clearance of disease-causing protein and other biomolecules by targeting them for lysosomal degradation using multi-functional molecules. The platform is differentiated from existing approaches in that it does not rely on ubiquitin ligases, and it allows for a broad range of targets to be degraded. About Biohaven Biohaven is a commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven's neuroinnovation portfolio includes FDA-approved NURTEC™ ODT (rimegepant) for the acute treatment of migraine and a broad pipeline of late-stage product candidates across three distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder, Alzheimer's disease, and spinocerebellar ataxia; and MPO inhibition for multiple system atrophy and amyotrophic lateral sclerosis. More information about Biohaven is available at www.biohavenpharma.com. Forward-Looking Statements This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "believe", "continue", "may", "will" and similar expressions, are intended to identify forward-looking statements. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of Biohaven's management about any of Biohaven's products or programs including, for example, NURTEC ODT as an acute treatment for patients with migraine and potential preventive treatment for migraine, Biohaven's pipeline of late-stage product candidates across three distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder, Alzheimer's disease, and spinocerebellar ataxia; and MPO inhibition for multiple system atrophy and amyotrophic lateral sclerosis, Kleo Pharmaceuticals compounds and programs or the Target Degrader (MoDEs) Platform licensed from Yale, the potential commercialization of Biohaven's product candidates, the potential for Biohaven's product candidates to be first in class or best in class therapies and the effectiveness and safety of Biohaven's product candidates. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of Biohaven's Annual Report on Form 10-K for the year ended December 31, 2019, filed with the Securities and Exchange Commission on February 26, 2020, and Biohaven's Quarterly Report on Form 10-Q for the quarter ended September 30, 2020. The forward-looking statements are made as of this date and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. Biohaven Contact: Business Development Contact for MATE, ARM or MoDE Platform Technologies: NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC. MATES and ARMS are trademarks of Kleo Pharmaceuticals, Inc. MoDEs is a trademark of Biohaven Therapeutics Ltd.
SOURCE Biohaven Pharmaceutical Holding Company Ltd. |
||
Company Codes: NYSE:BHVN |