| | - Reports new positive interim data from the ongoing Phase 1 study of BHV-1300, Biohaven’s lead investigational drug from its Molecular Degrader of Extracellular Proteins (MoDE™) platform
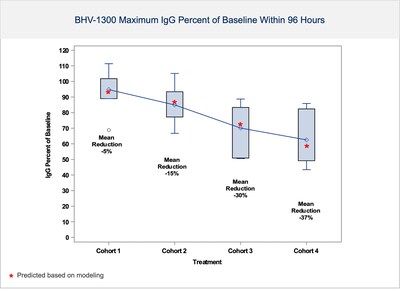
- BHV-1300 demonstrates dose-dependent and rapid IgG reductions within hours of administration
- No SAEs, no severe AEs, most AEs were mild, deemed unrelated to study drug and resolved spontaneously
- No clinically significant changes in LFTs across any dose cohorts to date
- Highlights advances from novel ion channel program targeting Kv7 activation and TRPM3 antagonism across multiple neurological, pain and neuropsychiatric disease indications
- Recently initiated a total of 5 pivotal clinical trials with selective Kv7 activator, BHV-7000, targeting focal epilepsy, idiopathic generalized epilepsy, bipolar disorder and major depressive disorder
- Released positive Phase 1 data with TRPM3 antagonist BHV-2100 showing drug concentrations above EC90 target and well-tolerated profile across all doses in SAD/MAD study; advancing Phase 2 study in migraine
- Anticipates Myostatin program topline data for Phase 3 Spinal Muscular Atrophy (SMA) study in 2H2024
- Reports new preclinical data highlighting potential for taldefgrobep alfa as monotherapy and in combination with GLP-1 agonists for weight loss:
- Taldefgrobep alfa in combination with GLP-1 in the diet-induced obesity preclinical model showed greater reductions in body weight and fat mass, and a larger increase in lean muscle mass, compared to GLP-1 alone
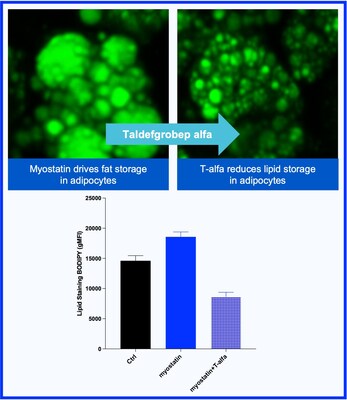
- Taldefgrobep alfa demonstrated direct effects on fat reduction as measured by changes in adipocytes independent of increasing muscle mass
- Releases positive Phase 1 data with BHV-8000, a brain-penetrant TYK2/JAK1 inhibitor, showing preliminary safety and achievement of target concentrations with reductions in inflammatory biomarkers in SAD/MAD study
- Key updates for BHV-8000 include favorable regulatory feedback enabling initiation of registrational programs for the prevention of ARIA associated with amyloid lowering drugs and Parkinson’s disease
- As announced in an earlier press release today, first patient dosed with Biohaven’s novel Trop-2 antibody drug conjugate (ADC), BHV-1510, in Phase 1/2 trial, as monotherapy and initiating combination with Regeneron’s anti-PDL1 Libtayo®(cemiplimab-rwlc), in advanced or metastatic epithelial tumors
NEW HAVEN, Conn., May 29, 2024 /PRNewswire/ -- Biohaven Ltd. (NYSE: BHVN) (Biohaven or the Company), a global clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of life-changing therapies, provides an overview of its development and regulatory advances across multiple therapeutic areas, and highlights the progress of its innovative degrader pipeline at the Company’s 2024 Investor R&D Day today, held concurrently with the Yale Innovation Summit in New Haven, Connecticut. Members of Biohaven’s senior management team and key opinion leaders will share updates with investors and research analysts. The presentation slides will be available on the Events and Presentations page of the Biohaven website. An audio webcast will be available within 24 hours of the presentation. The clinical progress, regulatory updates and pipeline developments at Biohaven’s R&D Day include: Molecular Degrader of Extracellular Proteins (MoDE) Platform: Harnessing a New Modality with Transformational Potential for the Treatment of Immunological and Inflammatory Disorders The Company unveiled new positive data from its ongoing Phase 1 single ascending dose (SAD) study with BHV-1300, a first-in-human IgG degrader that uses an ASGPR-bispecific from its MoDE platform. Emerging results in healthy subjects confirm that BHV-1300 rapidly and selectively lowers IgG in a dose-dependent manner in the first 4 cohorts completed to date (see Figure 1). Preliminary IgG lowering data is consistent with modeling, with dose- and time-dependent IgG lowering observed even in initial low-dose cohorts. Some subjects experienced IgG reductions as low as 50 to 70% of baseline. BHV-1300 demonstrated reduction of IgG without significantly impacting LFTs, albumin, LDL cholesterol or other serum labs. BHV-1300 has been safe and well tolerated to date, with no serious or severe adverse events. Most AEs were mild, deemed unrelated to study drug and resolved spontaneously. As expected from the selectivity of the molecule for IgG, when compared to placebo, there were no meaningful reductions in average IgA, IgM or IgE levels during the week after dosing. No adverse trends have been observed in vital signs or ECGs. Given the levels of IgG lowering observed to date, the company plans to evaluate approximately 6 cohorts of BHV-1300. Modeling suggests additional cohorts in the Phase 1 study will achieve > 70% lowering of IgG utilizing doses compatible with subcutaneous administration. Given the promising results of the SAD study thus far, the MAD study will proceed in patients with rheumatoid arthritis. - Advancing 3 additional novel MoDE degrader INDs on timelines for 2024
- Differentiated IgG degrader, BHV-1310, for myasthenia gravis
- Galactose-deficient IgA1 degrader, BHV-1400, for IgA nephropathy
- β1-AR autoantibody degrader for dilated cardiomyopathy
- Disclosing Additional Emerging Degrader Programs
- Biohaven disclosed additional novel MoDE degraders advancing to INDs including potential treatments for: 1) Type 1 diabetes with its degrader targeting anti-insulin and anti-proinsulin autoantibodies; 2) kidney disease with its degrader targeting phospholipase A2 receptor (PLA2R) antibodies for idiopathic membranous nephropathy; 3) IgG4 specific degrader to target IgG4-mediated rare diseases; and 4) gene therapy administration optimization with its degrader to target AAV9
- Multiple other degrader targets in development remain undisclosed.
Ion Channel Platform: Forging Much-Needed Novel Treatments for Patients with Neurological and Neuropsychiatric Disorders - Selective Kv7 Activator, BHV-7000, for Epilepsy, Bipolar Disorder, Major Depressive Disorder and Pain
- Initiated 5 pivotal clinical trials with BHV-7000, targeting focal epilepsy, generalized epilepsy, bipolar disorder and major depressive disorder. BHV-7000 offers the potential of a highly differentiated profile, having potent efficacy without burdensome central nervous system side effects. This furthers Biohaven’s goal of elevating the standard of care for these large indications with significant treatment gaps.
- Presented new data showing BHV-7000 attenuates action potential firing in inherited erythromelalgia (IEM) patient-derived sensory neuron induced pluripotent stem cells, suggesting potential to modify disease phenotype in patients with IEM and other pain disorders.
- Novel TRPM3 Antagonist, BHV-2100, for Migraine and Pain
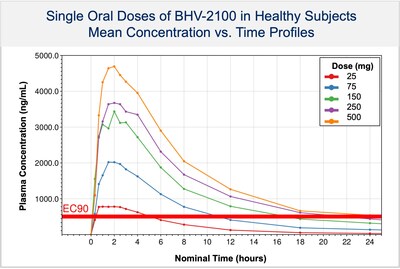
- Reported positive pharmacokinetic and safety data from the completed Phase 1 study with BHV-2100. The results demonstrate rapid absorption with therapeutic concentrations achieved by 20 minutes. The favorable tolerability profile at single doses up to 500 mg exceeds the anticipated therapeutic dose and is well above the EC90 concentration. These findings provide a compelling rationale for the advancement of BHV-2100 into clinical trials for both acute treatment of migraine and pain as a non-opiate therapy with minimal CNS side effects.
- Plans to initiate a Phase 2 study in acute treatment of migraine and a proof-of-concept study in pain in 2H2024.
Myostatin Program: Advancing an Innovative Approach for Improving Muscle Health - Myostatin Inhibitor, Taldefgrobep alfa, for Disrupting the Public Health Crisis of Obesity
- New preclinical data showing that administration of taldefgrobep alfa directly reduced the increased adipose fat storage caused by myostatin (see Figure 3).
- New preclinical data from a diet induced obesity mouse model, showed treatment with taldefgrobep alfa together with a GLP-1 agonists produced greater reductions in body weight and fat mass, and a larger increase in lean muscle mass, compared to treatment with GLP-1 alone. These data highlight the potential for taldefgrobep alfa to offer additional benefits, including enhancing muscle growth, when used in combination with a GLP-1. The Company plans to initiate a Phase 2 study in obesity in 2H2024.
- Myostatin Inhibitor, Taldefgrobep alfa, for Spinal Muscular Atrophy (SMA)
- Baseline characteristics of the population enrolled in the ongoing Phase 3 study in SMA were reported and confirmed to be well matched to the target clinical population. The primary endpoint of the study, the 32 Item Motor Function Measurement (MFM-32), is a reliable and validated endpoint for measuring clinically meaningful benefit in SMA. The MFM-32 lacks floor and ceiling effects, and has been used successfully in previous, registrational trials.
- Expect Phase 3 study top-line results in SMA in 2H 2024.
Neuroinflammation Platform: Selectively Targeting the Immune System to Treat Neurodegenerative Diseases - Brain-Penetrant TYK2/JAK1 Inhibitor, BHV-8000, for Prevention of Amyloid-Related Imaging Abnormalities (ARIA), Parkinson’s disease, Multiple Sclerosis and Alzheimer’s disease
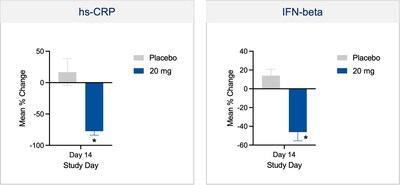
- Reported positive results from the Phase 1 single and multiple ascending dose study with BHV-8000 in healthy subjects, including evidence of target engagement (i.e., biomarker reductions in high-sensitivity C-reactive protein and interferon beta) along with a safe and well tolerated profile.
- Announced key regulatory updates, including the successful completion of two FDA meetings with favorable feedback enabling registrational programs for Parkinson’s disease and for the prevention of ARIA, a novel indication.
Glutamate Modulating Platform: Two Pivotal Trials in OCD and SCA Regulatory Workstreams Advance - Expect interim data analysis from second ongoing Phase 3 OCD trial in 4Q2024; Topline Data from first Phase 3 OCD trial expected in 1H2025.
- SCA filing in Europe continues in review and constructive interactions with FDA continue.
Oncology Platform: Building an Antibody Drug Conjugate (ADC) Franchise with Potential for Near- and Long-Term Value Creation - Next-Generation ADC portfolio leverages proprietary Biohaven MATETM technology platform
- Capable of generating a diverse and sustainable portfolio of highly differentiated ADCs
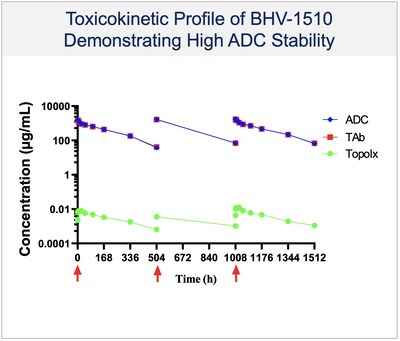
- The Company’s ADC candidates preclinically have demonstrated superior stability and improved efficacy, by optimizing on-target delivery and increasing therapeutic index.
- Biohaven plans to advance several programs into the clinic over the next 2-3 years.
- Novel Trop-2 ADC, BHV-1510, has entered into clinic for patients with advanced or metastatic epithelial tumors
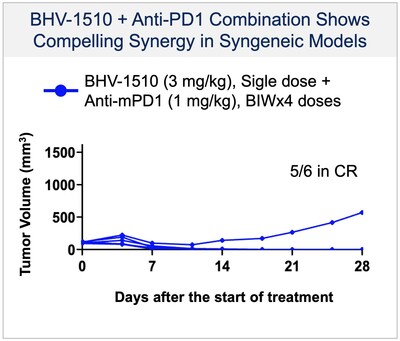
- BHV-1510 has demonstrated a highly differentiated preclinical monotherapy efficacy profile, the potential for broader therapeutic margin than other Trop-2 ADCs currently in development, and the potential for synergistic affects when combined with anti-PD1 therapy
- First patient was dosed in a Phase 1/2 clinical trial as monotherapy
- Biohaven also entered into a clinical supply agreement with Regeneron to study the combination of BHV-1510 with Regeneron’s anti-PD1 Libtayo (cemiplimab-rwlc) in the clinical study
Vlad Coric, M.D., Chairman and Chief Executive Officer of Biohaven, commented on the Company’s 2024 R&D Day: “Biohaven is leading the way in immune modulation with our first of its kind mechanism of action in MoDE degraders advancing through the early cohorts of Phase 1 testing. Equally important and exciting is that these data provide clinical validation for Biohaven’s MoDE degrader platform, which represents an entirely novel class of drugs with rapid development timelines and unlimited clinical and commercial potential. The platform can efficiently generate compounds designed to selectively degrade a specific extracellular protein of interest, such as an individual disease-causing autoantibody. The advancement of BHV-1300 has accelerated the development of other assets from the MoDE platform. We anticipate delivering approximately ten clinical-stage degraders over the next three years with the goal of radically transforming the treatment of a broad range of diseases, including up to three additional compounds by the end of this year. This technology has the potential to transform the treatment of autoimmune disorders and disrupt current treatment paradigms across therapeutic areas.” “In addition to our MoDE platform, we are advancing novel science in multiple therapeutics areas including ion channel modulation for neurological and neuropsychiatric indications, myostatin and activin modulation for muscle health and obesity, TYK2/JAK1 inhibition for neuroinflammatory conditions, glutamate modulation in neuroscience and a new generation of ADCs in oncology,” continued Dr. Coric. “I am so proud of the Biohaven team that is forging new scientific ground and working to improve the lives of patients not satisfied by current standard of care medications. Days matter for patients and their families, and the Biohaven team takes our responsibility seriously to efficiently move our programs forward to help those in need.” Bruce Car, DVM, Ph.D., DACVP, Chief Scientific Officer of Biohaven, commented, “We have built a high-performing team to tackle some of the most disabling diseases and conditions that face society. We are excited about the progress our R&D team is making in pursuing new druggable targets and disrupting older therapies with optimized technology with the goal of changing treatment paradigms. As we continue to advance our promising lead product candidates through upcoming milestones, our team will listen to the needs of patients and lean on our proven expertise in drug development execution to move with speed and efficiency on behalf of the millions of patients and families who are relying on our important work.” About Biohaven Biohaven is a biopharmaceutical company focused on the discovery, development, and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience and oncology. The company is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple proprietary drug development platforms. Biohaven’s extensive clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; TRPM3 antagonism for migraine and neuropathic pain; TYK2/JAK1 inhibition for neuroinflammatory disorders; glutamate modulation for OCD and SCA (spinocerebellar ataxia); myostatin inhibition for neuromuscular and metabolic diseases, including SMA and obesity; antibody recruiting bispecific molecules and antibody drug conjugates for cancer. For more information, visit www.biohaven.com. Forward-looking Statements This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including “continue”, “plan”, “will”, “believe”, “may”, “expect”, “anticipate” and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven’s planned and ongoing clinical trials; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; complying with applicable U.S. regulatory requirements; the potential commercialization of Biohaven’s product candidates; the potential for Biohaven’s product candidates to be first in class therapies; and the effectiveness and safety of Biohaven’s product candidates. Additional important factors to be considered in connection with forward-looking statements are described in Biohaven’s filings with the Securities and Exchange Commission, including within the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”. The forward-looking statements are made as of the date of this news release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. MoDE and MATE are trademarks of Biohaven Therapeutics Ltd.
Libtayo is a registered trademark of Regeneron Pharmaceuticals, Inc. Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
201-248-0741 Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
312-961-2502  View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-showcases-broad-innovative-portfolio-and-pipeline-updates-across-multiple-therapeutic-areas-including-immunology-neuroscience-metabolic-disorders-and-oncology-at-annual-investor-rd-day-302157949.html View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-showcases-broad-innovative-portfolio-and-pipeline-updates-across-multiple-therapeutic-areas-including-immunology-neuroscience-metabolic-disorders-and-oncology-at-annual-investor-rd-day-302157949.html
SOURCE Biohaven Ltd. | |