Werewolf Therapeutics is designing and developing molecules that unleash the natural ferocity of cytokines for the treatment of cancer.
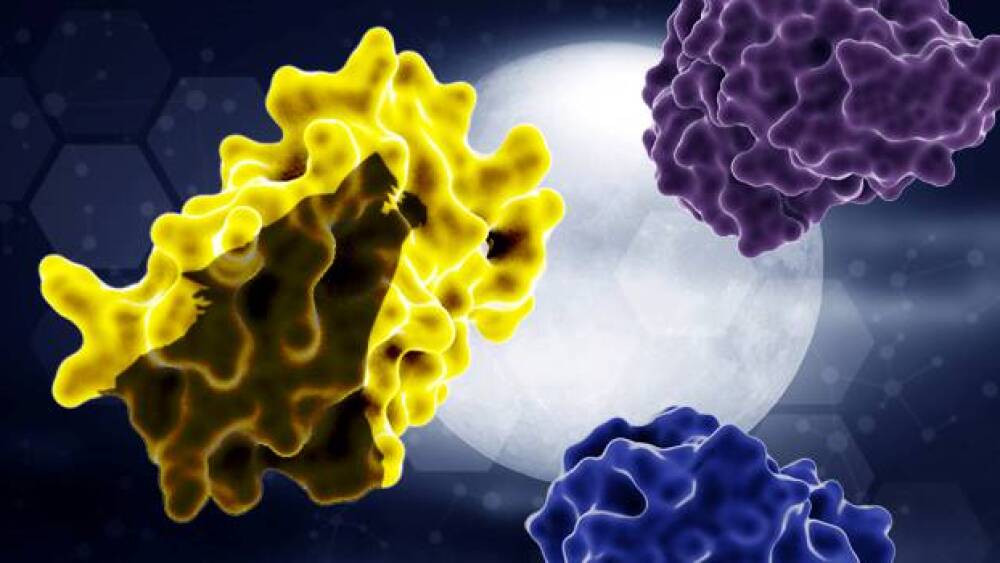
Tumor proteases activate the INDUKINETM molecule to release the inactivation domain (top-right) and half-life extension domain (bottom-right), unmasking a wild-type cytokine (left) that unleashes a powerful immune response within the tumor microenvironment.
Werewolf Therapeutics just might make you feel differently about cytokines. The Cambridge, Massachusetts-based oncology biotherapeutics company is designing and developing molecules that unleash the natural ferocity of cytokines for the treatment of cancer – but only when the “full moon” appears.
Cytokines have been in the news during the COVID-19 pandemic, in the context of a dangerous complication termed a “cytokine storm” that can result from viral infection. However, some of these immune-stimulating molecules are also clinically validated treatments for cancer that the industry has been seeking to develop into robust therapeutics for a number of years. Immuno-stimulatory cytokines are powerful signaling molecules, expressed rapidly upon immune cell activation, that can act within the tumor microenvironment to drive the proliferation and activation of key effector cells, thereby sending the body into attack mode against tumor antigens, which are recognized as “foreign”, similar to the response to viruses and other pathogens.
For those unfamiliar with the lore, werewolves are seemingly normal beings who circulate among the population, unrecognizable until the full moon appears. Then, they transform into killers.
“Our molecules are also designed to circulate in the body in an unrecognizable form,” Werewolf CSO Dr. Cynthia Seidel-Dugan, Ph.D. told BioSpace. “They’re not active, and then, once they reach the tumor microenvironment – i.e., the full moon – they are activated to become potent tumor killers.” These molecules, which Werewolf has engineered as conditionally-activated prodrugs, are called INDUKINE™ molecules.
Administered systemically, these wild-type cytokines circulate through the body as fully-masked prodrugs until they are released into the tumor and the tumor microenvironment at precisely the right moment.
“The masking components of our molecules are removed within the tumor microenvironment and the cytokine is then able to stimulate powerful anti-tumor activity by generating an immune response against the tumor cells,” Seidel-Dugan explained. In this way, Werewolf aims to overcome the limitations of current immuno-oncology therapies.
Interleukin-2 (IL-2), Interleukin-12 (IL-12) and Interferon alpha (IFNα) are potent stimulators of the immune system, but when free in the human body, their toxicities are difficult to manage because they stimulate the immune system in normal tissues as well. This means that “drug developers have historically been unable to really bring these therapies to a large number of patients,” Seidel-Dugan said.
Created with Werewolf’s proprietary PREDATOR™ protein engineering technology, INDUKINE molecules are designed to alleviate this barrier by remaining inactive until they reach the tumor microenvironment. “This will allow us to deliver these pro-inflammatory mechanisms only in the tumor while minimizing the systemic toxicities,” Seidel-Dugan added.
So, how can the molecules tell when the “full moon” appears?
Werewolf preserves the native, wild-type structure of the cytokine. It then attaches a high-affinity inactivation domain, which blocks the ability of the cytokine to bind to receptors in non-tumor tissue, and a half-life extension domain to improve its pharmaceutical properties given the wild-type cytokine’s very short half-life. The fourth key component is the proprietary protease cleavable linkers used during engineering to keep the components together within one prodrug protein structure.
“These linkers were selected because they’re very stable in normal tissues or in serum. In peripheral tissue, they are not cleaved, but then they can be selectively cleaved by tumor proteases which are overexpressed or dysregulated in the tumor microenvironment,” Seidel-Dugan explained. “That’s how we achieve the conditional activation of the molecule.”
Werewolf’s lead programs, WTX-124 and WTX-330, are both targeting investigational new drug (IND) applications in the first half of 2022. WTX-124 is a systemically-delivered, conditionally-activated Interleukin-2 (IL-2) INDUKINE molecule being developed initially for IO-sensitive tumors such as metastatic melanoma and renal cell cancers where recombinant IL-2 has been approved.
With this molecule, Werewolf has retained binding to the alpha subunit of the high-affinity IL-2 receptor, while engineering the INDUKINE molecule to block binding in its prodrug state to the medium affinity receptor and high-affinity IL-2 receptors in peripheral tissues.
“We think that this is a very powerful and optimal approach to addressing the toxicities associated with IL-2 and to deliver the maximum punch of IL-2 in the tumor microenvironment,” Seidel-Dugan shared.
This molecule is designed to finish the job on tumors where an immune response has already been mounted but hasn’t been completely successful. “That’s what IL-2 does best. It kickstarts cells that are not quite sure what to do and really kicks them into action to drive the anti-tumor activity,” Seidel-Dugan said.
In preclinical studies presented at The Society for Immunotherapy of Cancer (SITC) Annual Meeting in November, WTX-124 drove anti-tumor immunity in murine syngeneic cancer models.
Next up is WTX-330, a systemically-delivered, conditionally-activated Interleukin-12 (IL-12) INDUKINE molecule. This drug aims to overcome the severe toxicities that have been observed with recombinant IL-12 therapy and maximize the benefits.
“IL-12 has sort of been the holy grail of anti-cancer cytokine therapy,” Seidel-Dugan said, explaining that along with activating effector T cells, it can also stimulate some of the earliest stages of immune activation. The extreme toxicities associated with IL-12 have been especially prohibitive to treatment, she added. The precision approach taken by Werewolf seeks to “drive these anti-tumor immune responses, and to really probe the potential activity of this very exciting cytokine.”
In a poster presented at the 63rd American Society of Hematology (ASH) Annual Meeting in December, a WTX-330 surrogate inhibited syngeneic lymphoma tumor growth in mice, induced anti-tumor immune responses and was tolerated in non-human primates. Because it stimulates very early steps in the immune system, Werewolf believes this particular molecule “has the potential to even be active in less immunogenic tumors and has preclinical data to back up this hypothesis.”
“Unlike IL-2, where you need to already have an immune response started, we may have an opportunity here to treat patients who are refractory to checkpoint therapies or have tumors that are historically not sensitive to other IO therapies,” Seidel-Dugan said.
Coming up behind these two molecules in the pipeline is WTX-613, an INDUKINE molecule designed to overcome the limitations of Interferon alpha, a powerful stimulator of the innate immune response. IFNα has fallen out of favor therapeutically due to its own inherent toxicities. Werewolf is targeting the first half of 2023 to bring this one to an IND.
While Seidel-Dugan expressed confidence in the therapeutic targets Werewolf has pursued thus far, she shared that the company is keeping an eye out for new opportunities.
“We have a number of discovery programs which will look for additional immune mechanisms that we could potentially use our platform to target. There are certainly mechanisms that we have not yet tapped into that we would like to explore,” she said.
With 25 years of experience in the field, Seidel-Dugan is excited about the wide-open possibilities of immuno-oncology.
“It’s really become the fourth pillar of anti-cancer therapy, and I think there’s still a lot of opportunity for us. I think there are a lot of immune mechanisms that we still have an opportunity to exploit. Administering proinflammatory molecules is really an untapped mechanism,” she said. “We’ve only touched the tip of the iceberg.”
Featured Jobs on BioSpace