Brain cancer
While Imvax’s autologous immunotherapy IGV-001 missed the primary endpoint of progression-free survival in a Phase IIb trial, the company will request a meeting with the FDA to discuss next steps for “synergistic” treatment.
Arguably the FDA’s most anticipated decision this month is for a subcutaneous induction formulation of Biogen and Eisai’s Alzheimer’s drug Leqembi, which, according to Eisai, could “help reduce the burden on healthcare professionals and patients.”
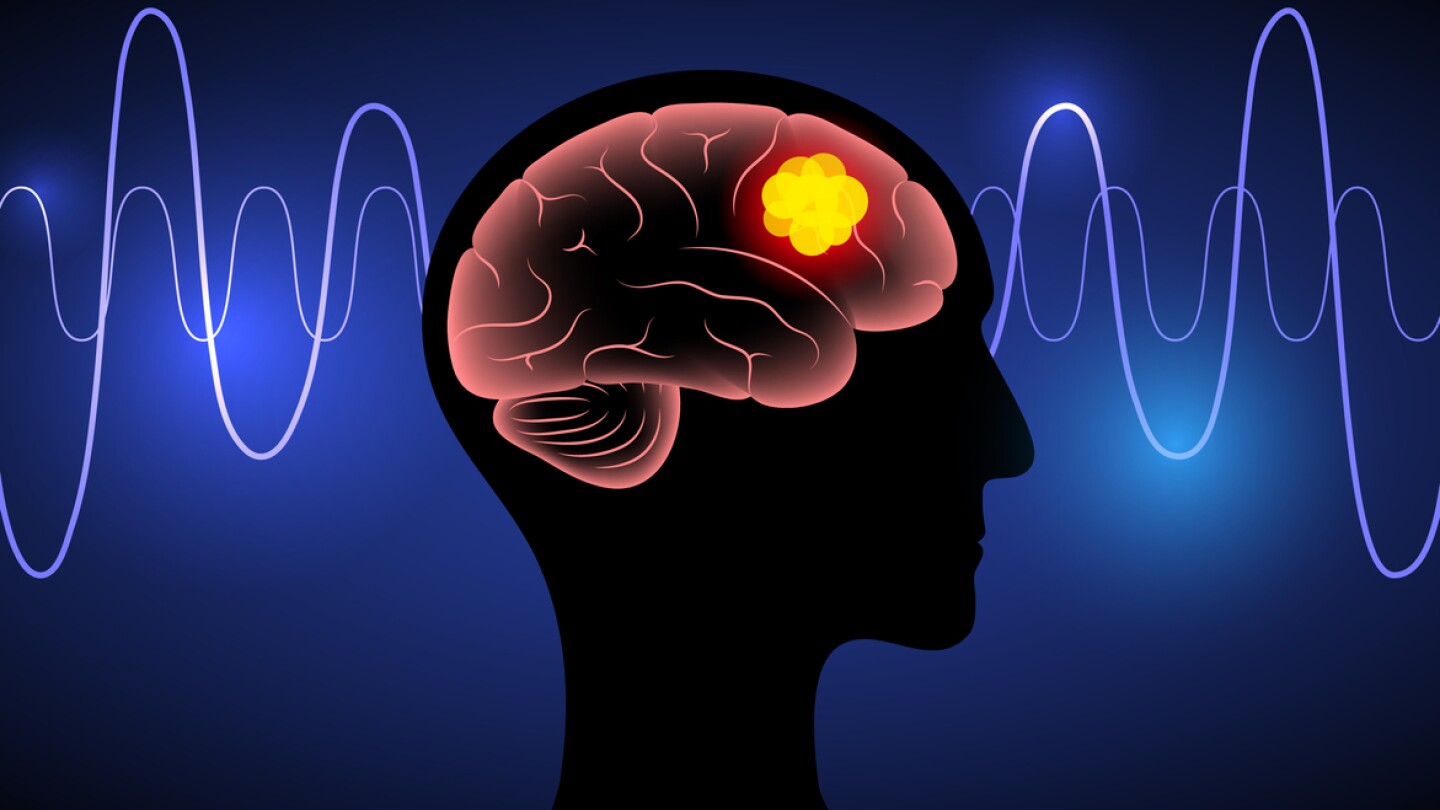
The small molecule drug, acquired by Jazz Pharmaceuticals in its $935 million Chimerix pick-up this spring, is intended for relapsed adult and pediatric patients with H3 K27M mutations.
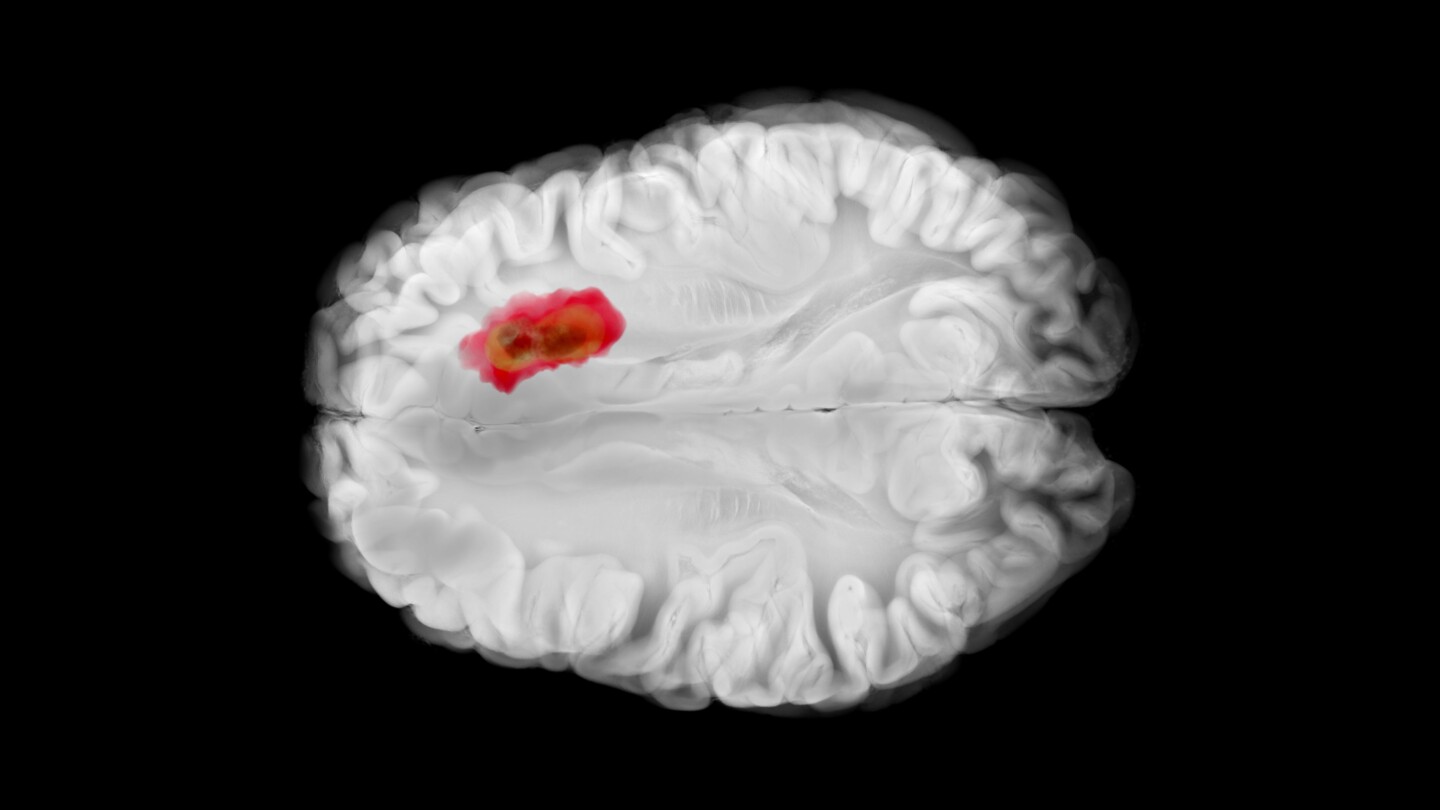
After decades of limited progress—owing to the difficulty of treating the disease and resultant market risk—glioblastoma research is entering a new phase spurred by smarter trials, targeted funding and renewed interest from companies like Merck and Jazz Pharmaceuticals.
Sanofi and BMS paid big money for rare disease and cancer assets, while Regeneron got in the obesity game; AstraZeneca, Gilead and Amgen shone at ASCO; RFK Jr. and the CDC appeared to disagree over COVID-19 vaccine recommendations and several news outlets are questioning the validity of the White House’s Make America Healthy Again report.
The medium-sized biopharma is showing off new results from dordaviprone and Zepzelca, both of which were acquired through Jazz Pharmaceuticals’ dealmaking over the last five years.
Stifel analysts were bullish on the data, which showed a 16.5% drop in body-mass index among patients with damage to the hypothalamus taking Rhythm Pharmaceuticals’ Imcivree.
At the heart of the deal is the drug candidate dordaviprone, which is months away from a regulatory verdict for its use in H3 K27M-mutated diffuse glioma.
Madrigal Pharmaceuticals, X4 Pharmaceuticals and Day One Biopharmaceuticals secured their maiden approvals this year in metabolic dysfunction-associated steatohepatitis, WHIM syndrome and pediatric low-grade glioma. Geron Corporation and ImmunityBio also notched wins.
Emboldened by technological advances and a deeper knowledge of glioblastoma, Merck, Kazia Therapeutics, CorriXR Therapeutics and others are targeting the often-fatal brain tumor.
PRESS RELEASES