As the industry awaits official word from the administration on how the tariffs will hit, analysts go over the possibilities with one certainty: there will be increased costs for medicines.
President Donald Trump is going to levy the pharmaceutical industry with tariffs despite broad objections to the plan. While he has not yet revealed the specifics, the industry is girding for potentially disruptive fees that will, according to many experts, do nothing to encourage onshoring of pharmaceutical manufacturing or make drugs cheaper.
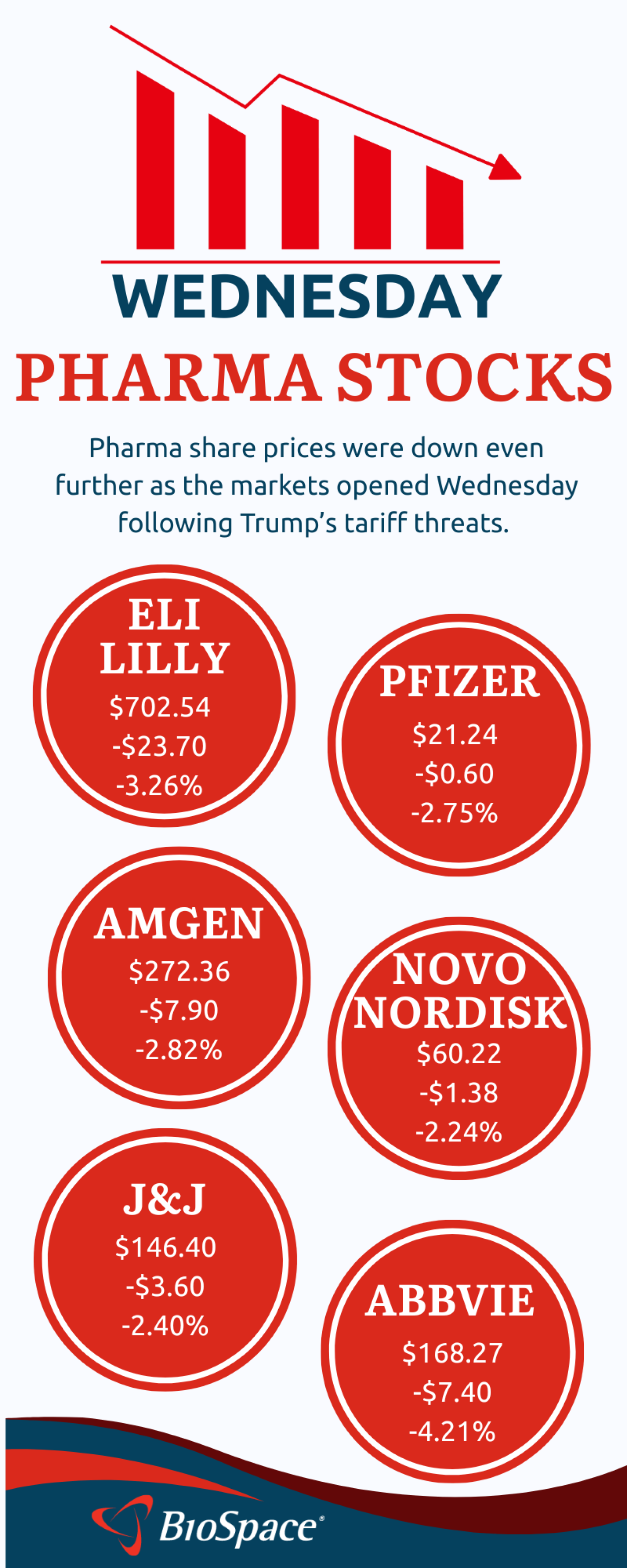
“Put on your life vest, as the waters are getting choppier,” BMO Capital Markets analysts warned in a note to investors early Wednesday morning.
As the industry awaits official word from the administration, pharma stocks are tanking, and analysts and other experts are considering the possible scenarios.
Arthur Wong, senior director of corporate ratings, healthcare, at S&P Global Ratings, pointed out that pharmas cannot just suddenly switch manufacturing to the U.S., even though most of the major companies have facilities in the country.
“It’s just a matter of how much capacity and how quickly can you switch that capacity to the U.S. so you could sidestep the tariff,” Wong told BioSpace.
There are a lot of unknowns at this point, but Wong said the tariffs will come down to what tax rate companies are charged as they move operations back to the U.S. “That’s the whole reason why they’re manufacturing [in] Ireland,” for example, he said.
BMO analysts agreed that pharmas do “capitalize on housing [intellectual property] in foreign jurisdictions.” But some companies like Eli Lilly and Vertex Pharmaceuticals have huge operations in the U.S. Lilly also just announced a $27 billion investment in manufacturing stateside. So did Johnson & Johnson, with a $55 billion commitment.
“It’s hard to believe that any administration would want to harm US companies, let alone US BioPharma, which is, in our view, an extremely enviable sector,” BMO wrote. “The US consistently has first access to the best medicines globally, reliable supply of any medicine. Having some supply chain overseas is good, as this adds redundancy/reliability.”
The industry had been working toward shifting some manufacturing outside of China due to pressures from Congress last year to do so. The BIOSECURE Act was to mandate a phase out of operations with certain contract manufacturing organizations like WuXi Biologics. While the legislation never passed—and as of now, has not been taken up again—the threat spooked biopharma into taking action.
But even if a pharma does have a facility capable of producing drugs in the U.S., finding the necessary staff to operate it will prove challenging, Wong said.
“Where are you going to hire these people from?” he wondered aloud. “The labor market is pretty tight within healthcare and to get people trained up and have that expertise brought up, that’s going to be difficult, and it’s going to take time to get the full efficiency.”
Wong pointed to the semiconductor industry, which has been similarly challenged to bring manufacturing to the U.S. “They have to bring people over from Taiwan into the States,” he said. “So do you do the same thing with bringing people over from Ireland [for pharma manufacturing]?”
Treasury Secretary Scott Bessent on Monday offered a solution: hire laid off federal workers to staff manufacturing jobs. But Wong says these jobs are highly skilled and take time to train for.
“I’m not saying you can’t find it, but it’s going to take time to hire up and it’s going to take time to train,” Wong said. “It’s going to take time to get to that higher level efficiency. I gotta think you have to bring people over from overseas.”
BMO agreed. “Global supply chains are complex, with Pharma among the most,” the analysts wrote—"it’s not as simple as moving where someone screws in little screws to make an iPhone.”
Running Out the Clock
Another key question is whether the tariffs will be based on the cost of goods, including raw materials, labor and direct factory overheads. Other uncertainties include the tariff rate, whether the fees will be phased in or dumped whole hog, and what products they will apply to.
Tariffs could hit big, branded biologics and high-margin products or lower cost generics, Wong said. Both options would be a big blow to the industry. Generics make up about 90% of prescriptions written in the U.S., while biologics and brand-name drugs make the most revenue.
For the high-margin branded products, Wong said, “there’s going to be some cost-cutting, and it’s going to take time to reabsorb that hit on the tariff.” Pharmas have more leeway to pass the costs on to customers, he added. On the generics side, pharmas have little wiggle room to raise prices and absorb the tariff cost because of the “commodity-like nature” of the market, according to a recent S&P Global Ratings analysis.
Ultimately, a lot of active pharmaceutical ingredients (API) still come from overseas, China in particular. Trump has particularly targeted China with the highest tariff rates and pharmaceuticals have not been excluded from that. If a 10% tariff is enacted on API from China, S&P estimated that a generics company would take a 2% to 3% hit on its profit margins. If companies do seek alternative sources for these drug ingredients, S&P worried about supply disruptions and an exacerbation of drug shortages.
Wong noted that companies sign long-term contracts for these ingredients; they can’t just sever agreements on a whim. The BMO group said that often manufacturing facilities are situated near the source of ingredients to ease the production process.
With all these factors to consider and the time to implement any shifts in production, BMO speculates that pharma is simply going to wait out the changes brought by the new administration. “We expect most large pharma’s are likely to look at imposed tariffs with the intention of ‘running out the clock,’ waiting until the end of Trump’s presidency to consider more permanent manufacturing decisions.”
While innovative, unapproved products likely won’t be directly targeted, S&P noted that the tariffs could put a chilling effect on the pharma and life-science industries, impacting clinical trials and patient data. This could lead to delays in new drug development.
And Wong said that all this chaos is going “put a pause [on] or distract” from another key pharma function: dealmaking. With biotech valuations still declining as the industry continued to suffer from a prolonged downturn following the COVID-19 pandemic, the moment to execute on M&A had arrived. But now Wong predicts we won’t see a return of deals until the second half of 2025. “Hopefully, things quiet down a bit,” he said.