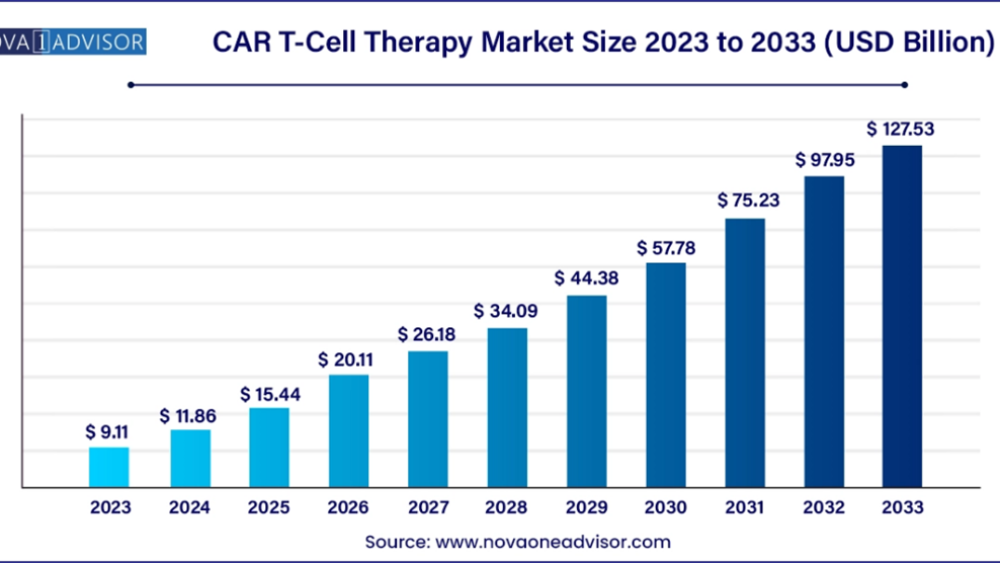
According to Nova One Advisor, the global CAR T-Cell therapy market size was valued at USD 9.11 Billion in 2023 and is expected to grow from USD 11.86 Billion in 2024 to reach USD 127.53 billion by 2033, at a CAGR of 30.2% during the forecast period (2024-2033)
Significant growth factor for CAR T-cell therapy is its short treatment duration, typically involving a single infusion and up to two weeks of inpatient care, contrasting with the prolonged recovery associated with aggressive chemotherapy used in stem cell transplants. This approach facilitates quicker patient recovery, enhancing treatment feasibility and patient acceptance. CAR T-cell therapy continues to demonstrate efficacy in patients for whom traditional transplants are not curative or who experience relapse post-transplant, its adoption is poised to expand.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/6000
Market Overview
CAR T-cell therapy represents a transformative advancement in cancer immunotherapy, leveraging genetically modified T cells to target and destroy cancer cells more effectively. This innovative treatment, utilizing chimeric antigen receptor (CAR) technology, is primarily employed for treating certain types of blood cancers that have proven resistant to conventional therapies or have recurred. CAR T-cell therapy not only offers the potential for curing specific blood cancers but also extends survival and achieves long-term remissions in some patients. Its ability to persist in the body after infusion contributes to sustained therapeutic benefits. As a result, CAR T-cell therapy has rapidly emerged as a promising approach in oncological care, driving significant growth in the market as healthcare providers increasingly adopt this groundbreaking treatment for patients in need.
§ In February 2024, BioNTech and Autolus announced a strategic CAR-T cell therapy collaboration to advance their pipeline and expand late-stage programs.
§ In December 2023, AstraZeneca announced plans to acquire Gracell, furthering its cell therapy ambitions across oncology and autoimmune diseases.
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/6000
CAR T-Cell Therapy Market Key Takeaways
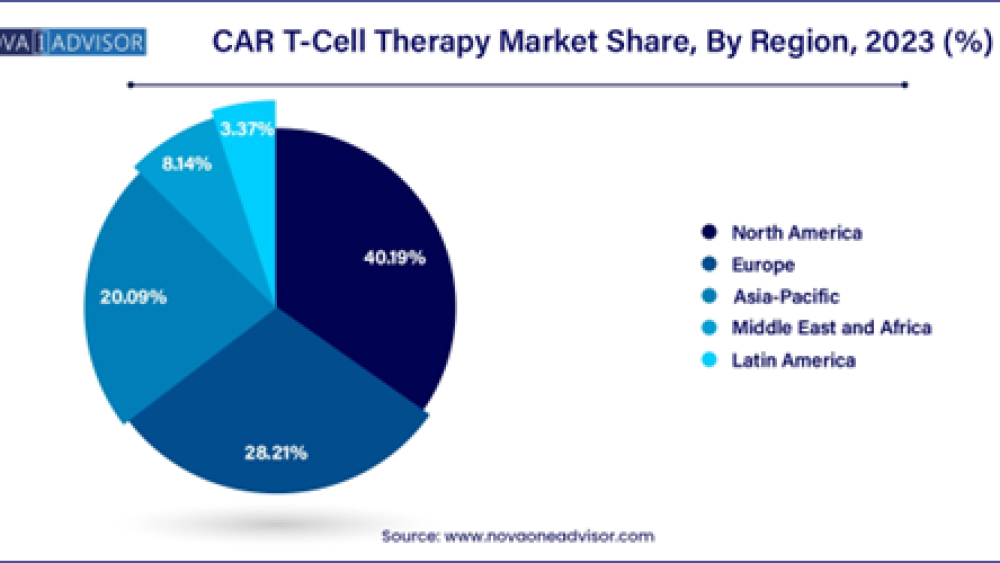
· Europe CAR T-cell therapy market size was exhibited at USD 2.37 billion in 2023 and it is expanding around USD 29.82 billion by 2033 with a CAGR of 30.3% from 2024 to 2033.
· APAC CAR T-cell therapy market size was surpassed at USD 1.69 billion in 2023 and it is projected to reach around USD 21.65 billion by 2033, expanding at a CAGR of 30.5% from 2024 to 2033.
· LAMEA CAR T-cell therapy market size was valued at USD 1.01 billion in 2023 and it is predicted to reach around USD 10.54 billion by 2033 with a CAGR of 26.8% from 2024 to 2033.
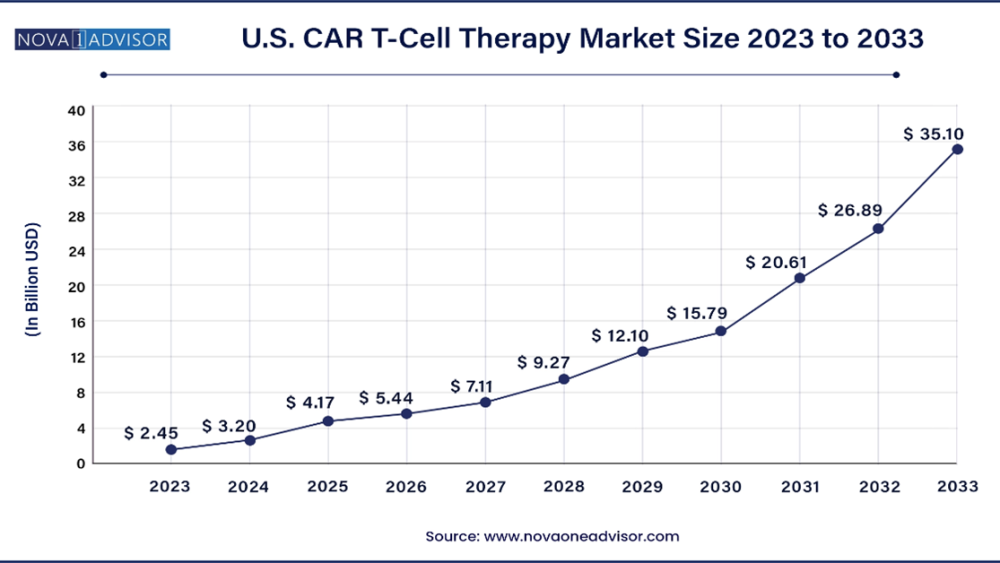
U.S. CAR T-Cell Therapy Market Size, Share, Growth Report, 2033
The U.S. CAR T-Cell Therapy market is valued at USD 2.45 Billion in 2023 and is projected to reach a value of USD 35.10 Billion by 2033 at a CAGR (Compound Annual Growth Rate) of 30.5% between 2023 and 2033.
North America currently leads the CAR T-cell therapy market, driven by significant clinical efficacy demonstrated in trials and the commercial approval of various products by regulatory agencies in the USA. Efforts are underway to expand access to CAR-T cell therapy in Mexico, reflecting ongoing regional adoption and development efforts. In Canada, the Conconi Family Immunotherapy Lab at BC Cancer’s Deeley Research Centre has pioneered CAR T-cell production, establishing critical infrastructure for advancing immunotherapy research.
The National Research Council (NRC) collaborates with academic, clinical, and industry partners to develop indigenous CAR T-cell therapies, focusing on affordability and accessibility for Canadian patients. These initiatives underscore North America's pivotal role in advancing and integrating CAR T-cell therapies into mainstream oncological care, driving regional growth and innovation in the market.
§ In March 2024, the U.S. FDA approved Bristol Myers Squibb’s Breyanzi as the first and only CAR T cell therapy for adults with relapsed or refractory Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). The Asia Pacific CAR T-cell therapy market is poised for substantial growth, supported by significant regulatory advancements and clinical efficacy demonstrations.
§ In October 2023, India's Central Drugs Standard Control Organization approved actaly-cel, marking the country's first approval of a CAR T-cell therapy for relapsed or refractory B-cell lymphomas and leukemia. This milestone underscores India's increasing integration of advanced immunotherapies into clinical practice. In China, CAR T-cell therapy has been incorporated into treatment protocols for hematologic malignancies, including leukemia, lymphoma, and multiple myeloma (MM), where it has shown efficacy.
Challenges persist, with significant relapse rates reported post-therapy. These challenges, ongoing research and regulatory efforts in the region are expected to drive continued expansion and accessibility of CAR T-cell therapies, positioning Asia Pacific as a pivotal market for future advancements in cellular immunotherapy.
CAR T-Cell Therapy Market Revenue, By Regions, 2020 to 2023 (US$ Million)
Buy Full Report: https://www.novaoneadvisor.com/report/checkout/6000
Report Highlights
By Drug Type
Axicabtagene ciloleucel (axi-cel) emerges as a dominant drug type in the global CAR T-cell therapy market, recognized for its efficacy in treating refractory large B-cell lymphoma. As an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, axi-cel has demonstrated effectiveness in patients whose disease persists or recurs after multiple lines of systemic therapy. Approved for use in adults with relapsed or refractory follicular lymphoma and certain types of large B-cell lymphoma, axi-cel's therapeutic potential extends to ongoing research exploring its application in other cancer types. This drug type's prominence underscores its pivotal role in addressing unmet medical needs and driving advancements in CAR T-cell therapy globally.
Tisagenlecleucel is anticipated to exhibit the highest growth rate in the forecast period within the CAR T-cell therapy market. As the first FDA-approved chimeric antigen receptor (CAR) T-cell therapy, tisagenlecleucel represents a significant advancement in cancer treatment. Initially approved for adults with certain types of B-cell non-Hodgkin lymphoma and young individuals up to 25 years old with B-cell acute lymphoblastic leukemia, tisagenlecleucel continues to be studied for its potential application in other cancer types. This drug type's rapid growth is driven by its pioneering status, clinical efficacy, and ongoing research initiatives aimed at expanding its therapeutic footprint, positioning it as a pivotal segment in the evolving landscape of CAR T-cell therapies.
CAR T-Cell Therapy Market Revenue, By Drug Type, 2020 to 2023 (US$ Million)
By Indication
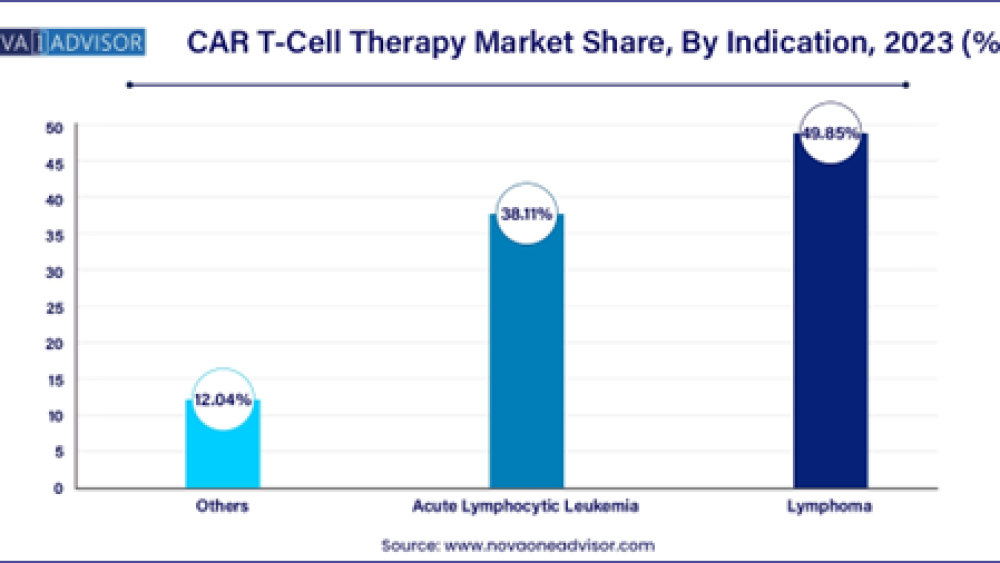
The lymphoma segment holds a dominant position in the global CAR T-cell therapy market and is poised for growth in the projected period. This growth is primarily driven by the rising incidence of non-Hodgkin lymphoma cases worldwide. CAR T-cell therapy, leveraging chimeric antigen receptor (CAR) technology, harnesses patients' immune cells to target and eliminate cancerous lymphoma cells. Engineered CAR molecules enhance immune response by recognizing specific antigens on lymphoma cell surfaces, facilitating targeted therapy. As advancements continue in CAR T-cell technology and clinical applications, the lymphoma segment remains pivotal in shaping the evolving landscape of oncological treatment options, underscoring its significant role and potential growth in the market.
The acute lymphocytic leukemia (ALL) segment is poised to experience rapid growth, driven by its challenging prognosis, particularly in cases of relapsed or refractory disease. T-cell acute lymphoblastic leukemia (T-ALL) presents fewer treatment options compared to B-cell ALL, necessitating innovative approaches like CAR T-cell therapy. Using autologous CAR T-cells in T-ALL is complicated by shared antigens with normal T-cells, leading to fratricide and potential T-cell aplasia if CAR T-cells persist. Contamination risk of the apheresis product by lymphoblasts further complicates therapy. To mitigate these challenges, various T-cell editing methods, such as protein expression blockers, CRISPR/Cas9, and base-editing, are employed. These advancements in T-cell engineering aim to enhance CAR T-cell therapy efficacy and safety, addressing critical unmet needs in treating T-ALL and driving growth in this segment of the CAR T-cell therapy market.
CAR T-Cell Therapy Market Revenue, By Indication, 2020 to 2023 (US$ Million)
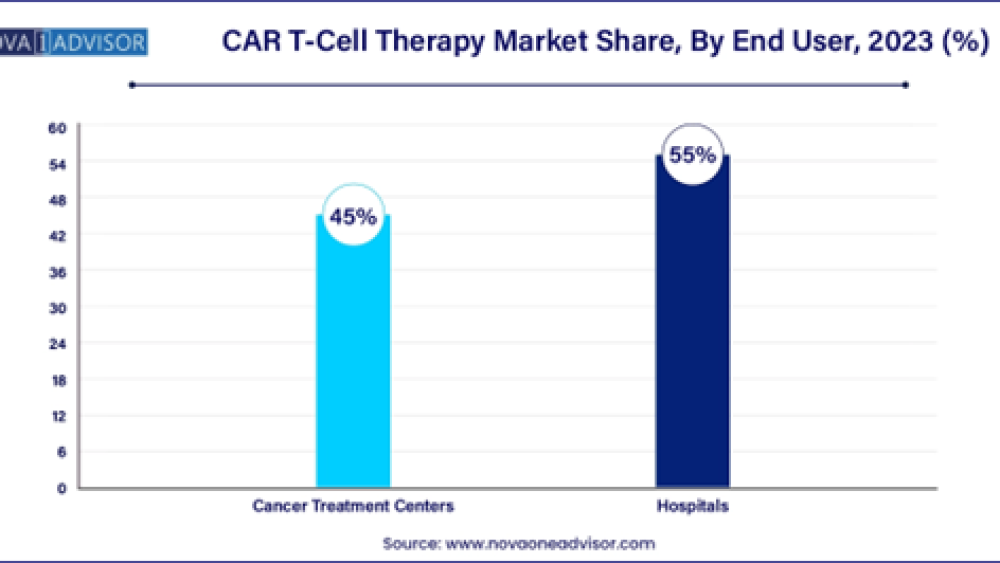
By End User
The hospital segment holds the largest market share in the global CAR T-cell therapy market, driven by its role in managing patients undergoing this advanced cancer treatment. CAR T-cell therapy recipients typically undergo a risk and recovery period lasting approximately 2 to 3 months post-treatment, during which they are closely monitored for treatment response and potential side effects. Hospital admissions are common during this period to manage and mitigate complications that may arise from the therapy. This underscores hospitals' pivotal role as primary care providers and treatment centers for CAR T-cell therapy patients, supporting their comprehensive medical needs from initial treatment through recovery phases.
The cancer treatment center segment is expected to experience the highest compound annual growth rate (CAGR) during the forecast period in the global CAR T-cell therapy market. These centers play a crucial role in advancing CAR T-cell therapy through basic laboratory research, clinical trials, and epidemiological studies aimed at understanding cancer patterns, causes, and management strategies across diverse patient groups. Their involvement in multicenter clinical trials facilitates collaboration and patient enrollment from various regions, enhancing the breadth and depth of research outcomes. As pivotal hubs for oncological research and patient care, cancer treatment centers contribute significantly to shaping the future of CAR T-cell therapy, driving innovation and expanding treatment options for cancer patients globally.
CAR T-Cell Therapy Market Revenue, By End User, 2020 to 2023 (US$ Million)
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/6000
Market Dynamics
Driver
Clinical Success and Advancements in CAR T-Cell Therapy
CAR T-cell therapy represents a pioneering approach to treating refractory blood cancers, offering a potential cure where conventional treatments have faltered. Initially developed for acute lymphoblastic leukemia (ALL), particularly in pediatric cases where intensive chemotherapy achieves high cure rates for B-cell types. Challenges persist for patients experiencing relapses post-chemotherapy or stem-cell transplant, highlighting the critical need for effective alternatives. CAR T-cell therapies address this gap by harnessing the body's immune system to target cancer cells, showcasing significant clinical successes in achieving remission and potential cures. These advancements drive optimism and market growth potential for CAR T-cell therapies, positioning them as transformative treatments in oncology.
In May 2024, Astellas and Poseida Therapeutics entered into a research collaboration and license agreement to develop novel allogeneic cell therapies in oncology.
Restraint
Side Effects and Care Requirements
CAR T-cell therapy can be highly effective against some types of hard-to-treat cancers, it also presents significant challenges that may constrain market growth. The therapy can cause serious or even life-threatening side effects, necessitating administration in specialized medical centers with trained staff. Patients require close monitoring for several weeks post-treatment to manage potential complications.
One major side effect is Cytokine Release Syndrome (CRS), where multiplying CAR T cells release large amounts of cytokines into the blood, excessively stimulating the immune system. This can lead to severe side effects, including high fever and chills, trouble breathing, severe nausea, vomiting, diarrhea, dizziness, lightheadedness, and headaches. These risks and the need for specialized care facilities limit the widespread adoption and growth of the CAR T-cell therapy market.
Opportunity
Expanding Applications of CAR-T Cell Therapy
Modern trends and recent developments in CAR-T cell therapy are increasingly focusing on diseases beyond cancer, such as autoimmune disorders and viral infections, including SARS-CoV-2. The number of available targets for CAR-T cell therapy is rapidly expanding, driven by the growing understanding of various pathologies and their fundamental molecular mechanisms. This progress is pushing the application boundaries of cellular immunotherapy far beyond oncology.
CAR-T cell technology holds great promise for treating viral infections in patients with primary immune deficiency (PID). Ongoing research in this field offers hope for many currently incurable diseases, potentially leading to novel CAR-T cell therapies and paving the way for future breakthroughs in clinical applications. These advancements create significant growth opportunities for the CAR-T cell therapy market.
Immediate Delivery Available, Get Full Access@ https://www.novaoneadvisor.com/report/checkout/6000
CAR T-Cell Therapy Market Top Key Companies:
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the CAR T-Cell Therapy market.
By Drug Type
https://www.novaoneadvisor.com/report/checkout/6000
Key Benefits for Stakeholders
Cell Therapy Market Size and Forecast: The global cell therapy market size was exhibited at USD 4.85 billion in 2023 and is projected to hit around USD 37.42 billion by 2033, growing at a CAGR of 22.67% during the forecast period 2024 to 2033.
Gene Therapy Market Size and Forecast : The global gene therapy market size was exhibited at USD 8.75 billion in 2023 and is projected to hit around USD 52.40 billion by 2033, growing at a CAGR of 19.6% during the forecast period 2024 to 2033.
Cell And Gene Therapy Manufacturing Market Size and Forecast: The global cell and gene therapy manufacturing market size was estimated at USD 9.95 billion in 2023 and is projected to hit around USD 106.03 billion by 2033, growing at a CAGR of 26.7% during the forecast period from 2024 to 2033.
Cell and Gene Therapy Market Size and Forecast: The global cell and gene therapy market size was estimated at USD 18.13 billion in 2023 and is projected to hit around USD 97.33 billion by 2033, growing at a CAGR of 18.3% during the forecast period from 2024 to 2033.
U.S. Cell Therapy Market Size and Forecast: The U.S. cell therapy market size was estimated at USD 2.88 billion in 2023 and is projected to hit around USD 19.67 billion by 2033, growing at a CAGR of 21.18% during the forecast period from 2024 to 2033.
U.S. Gene Therapy Market Size and Forecast: The U.S. gene therapy market size was estimated at USD 3.19 billion in 2023 and is projected to hit around USD 18.50 billion by 2033, growing at a CAGR of 19.22% during the forecast period from 2024 to 2033.
T-cell Therapy Market Size and Forecast: The global T-Cell therapy market size was exhibited at USD 3.85 billion in 2023 and is projected to hit around USD 79.62 billion by 2033, growing at a CAGR of 35.38% during the forecast period 2024 to 2033.
Cancer Immunotherapy Market Size and Forecast: The global cancer immunotherapy market size was valued at USD 126.19 billion in 2023 and is projected to surpass around USD 296.01 billion by 2033, registering a CAGR of 8.9% over the forecast period of 2024 to 2033.
Immunotherapy Drugs Market : The global immunotherapy drugs market size was valued at USD 240.19 billion in 2023 and is projected to surpass around USD 1,300.38 billion by 2033, registering a CAGR of 18.4% over the forecast period of 2024 to 2033.
Clinical Trials Market Size and Forecast: The global clinical trials market size was estimated at USD 81.90 billion in 2023 and is projected to hit around USD 153.59 billion by 2033, growing at a CAGR of 6.49% during the forecast period from 2024 to 2033.
Biologics Market Size and Forecast: The global biologics market size was estimated at USD 511.04 billion in 2023 and is projected to hit around USD 1,374.51 billion by 2033, growing at a CAGR of 10.4% during the forecast period from 2024 to 2033.
Biotechnology Market Size and Forecast: The global biotechnology market size was estimated at USD 1.54 Trillion in 2023 and is projected to hit around USD 5.68 Trillion by 2033, growing at a CAGR of 13.95% during the forecast period from 2024 to 2033.
Oncology Market Size and Forecast: The global oncology market size was estimated at USD 222.36 billion in 2023 and is projected to hit around USD 521.60 billion by 2033, growing at a CAGR of 8.9% during the forecast period from 2024 to 2033.
Cell And Gene Therapy Clinical Trials Market Size and Forecast: The global cell and gene therapy clinical trials market size reached USD 11.62 billion in 2023 and is projected to hit around USD 47.40 billion by 2033, expanding at a CAGR of 15.09% during the forecast period from 2024 to 2033.
U.S. Clinical Trials Market Size and Forecast: The U.S. clinical trials market size was valued at USD 25.81 billion in 2023 and is projected to surpass around USD 41.57 billion by 2033, registering a CAGR of 4.88% over the forecast period of 2024 to 2033.
Procure Complete Report (220+ Pages PDF with Insights, Charts, Tables, and Figures) @
https://www.novaoneadvisor.com/report/checkout/6000
About Us
Nova One Advisor is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Nova One Advisor has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defines, among different ventures present globally.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Email: sales@novaoneadvisor.com
Web: https://www.novaoneadvisor.com/
Significant growth factor for CAR T-cell therapy is its short treatment duration, typically involving a single infusion and up to two weeks of inpatient care, contrasting with the prolonged recovery associated with aggressive chemotherapy used in stem cell transplants. This approach facilitates quicker patient recovery, enhancing treatment feasibility and patient acceptance. CAR T-cell therapy continues to demonstrate efficacy in patients for whom traditional transplants are not curative or who experience relapse post-transplant, its adoption is poised to expand.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/6000
Market Overview
CAR T-cell therapy represents a transformative advancement in cancer immunotherapy, leveraging genetically modified T cells to target and destroy cancer cells more effectively. This innovative treatment, utilizing chimeric antigen receptor (CAR) technology, is primarily employed for treating certain types of blood cancers that have proven resistant to conventional therapies or have recurred. CAR T-cell therapy not only offers the potential for curing specific blood cancers but also extends survival and achieves long-term remissions in some patients. Its ability to persist in the body after infusion contributes to sustained therapeutic benefits. As a result, CAR T-cell therapy has rapidly emerged as a promising approach in oncological care, driving significant growth in the market as healthcare providers increasingly adopt this groundbreaking treatment for patients in need.
§ In February 2024, BioNTech and Autolus announced a strategic CAR-T cell therapy collaboration to advance their pipeline and expand late-stage programs.
§ In December 2023, AstraZeneca announced plans to acquire Gracell, furthering its cell therapy ambitions across oncology and autoimmune diseases.
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/6000
CAR T-Cell Therapy Market Key Takeaways
- The North America dominates the market with revenue share of 40.19% in 2023.
- By drug type, the axicabtagene ciloleucel segment dominate the market with revenue share of 29.28% in 2023.
- By indication, the lymphoma segment has accounted revenue share of 49.85% in 2023.
- By end user, the hospital segment led the market and contributed more than 55% of revenue share in 2023.
· Europe CAR T-cell therapy market size was exhibited at USD 2.37 billion in 2023 and it is expanding around USD 29.82 billion by 2033 with a CAGR of 30.3% from 2024 to 2033.
· APAC CAR T-cell therapy market size was surpassed at USD 1.69 billion in 2023 and it is projected to reach around USD 21.65 billion by 2033, expanding at a CAGR of 30.5% from 2024 to 2033.
· LAMEA CAR T-cell therapy market size was valued at USD 1.01 billion in 2023 and it is predicted to reach around USD 10.54 billion by 2033 with a CAGR of 26.8% from 2024 to 2033.
U.S. CAR T-Cell Therapy Market Size, Share, Growth Report, 2033
The U.S. CAR T-Cell Therapy market is valued at USD 2.45 Billion in 2023 and is projected to reach a value of USD 35.10 Billion by 2033 at a CAGR (Compound Annual Growth Rate) of 30.5% between 2023 and 2033.
North America currently leads the CAR T-cell therapy market, driven by significant clinical efficacy demonstrated in trials and the commercial approval of various products by regulatory agencies in the USA. Efforts are underway to expand access to CAR-T cell therapy in Mexico, reflecting ongoing regional adoption and development efforts. In Canada, the Conconi Family Immunotherapy Lab at BC Cancer’s Deeley Research Centre has pioneered CAR T-cell production, establishing critical infrastructure for advancing immunotherapy research.
The National Research Council (NRC) collaborates with academic, clinical, and industry partners to develop indigenous CAR T-cell therapies, focusing on affordability and accessibility for Canadian patients. These initiatives underscore North America's pivotal role in advancing and integrating CAR T-cell therapies into mainstream oncological care, driving regional growth and innovation in the market.
§ In March 2024, the U.S. FDA approved Bristol Myers Squibb’s Breyanzi as the first and only CAR T cell therapy for adults with relapsed or refractory Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). The Asia Pacific CAR T-cell therapy market is poised for substantial growth, supported by significant regulatory advancements and clinical efficacy demonstrations.
§ In October 2023, India's Central Drugs Standard Control Organization approved actaly-cel, marking the country's first approval of a CAR T-cell therapy for relapsed or refractory B-cell lymphomas and leukemia. This milestone underscores India's increasing integration of advanced immunotherapies into clinical practice. In China, CAR T-cell therapy has been incorporated into treatment protocols for hematologic malignancies, including leukemia, lymphoma, and multiple myeloma (MM), where it has shown efficacy.
Challenges persist, with significant relapse rates reported post-therapy. These challenges, ongoing research and regulatory efforts in the region are expected to drive continued expansion and accessibility of CAR T-cell therapies, positioning Asia Pacific as a pivotal market for future advancements in cellular immunotherapy.
CAR T-Cell Therapy Market Revenue, By Regions, 2020 to 2023 (US$ Million)
| CAR T-Cell Therapy Market Revenue, 2020-2023 (US$ Million) | ||||
| By Regions | 2020 | 2021 | 2022 | 2023 |
| North America | 438.0 | 739.1 | 1,521.9 | 3,361.1 |
| Europe | 307.3 | 520.1 | 1,074.2 | 2,379.5 |
| Asia Pacific | 217.7 | 369.0 | 763.4 | 1,693.7 |
| LAMEA | 140.3 | 231.9 | 467.5 | 1,010.3 |
Buy Full Report: https://www.novaoneadvisor.com/report/checkout/6000
Report Highlights
By Drug Type
Axicabtagene ciloleucel (axi-cel) emerges as a dominant drug type in the global CAR T-cell therapy market, recognized for its efficacy in treating refractory large B-cell lymphoma. As an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, axi-cel has demonstrated effectiveness in patients whose disease persists or recurs after multiple lines of systemic therapy. Approved for use in adults with relapsed or refractory follicular lymphoma and certain types of large B-cell lymphoma, axi-cel's therapeutic potential extends to ongoing research exploring its application in other cancer types. This drug type's prominence underscores its pivotal role in addressing unmet medical needs and driving advancements in CAR T-cell therapy globally.
Tisagenlecleucel is anticipated to exhibit the highest growth rate in the forecast period within the CAR T-cell therapy market. As the first FDA-approved chimeric antigen receptor (CAR) T-cell therapy, tisagenlecleucel represents a significant advancement in cancer treatment. Initially approved for adults with certain types of B-cell non-Hodgkin lymphoma and young individuals up to 25 years old with B-cell acute lymphoblastic leukemia, tisagenlecleucel continues to be studied for its potential application in other cancer types. This drug type's rapid growth is driven by its pioneering status, clinical efficacy, and ongoing research initiatives aimed at expanding its therapeutic footprint, positioning it as a pivotal segment in the evolving landscape of CAR T-cell therapies.
CAR T-Cell Therapy Market Revenue, By Drug Type, 2020 to 2023 (US$ Million)
| Drug Type | 2020 | 2021 | 2022 | 2023 |
| Axicabtagene Ciloleucel | 321.2 | 542.5 | 1,118.3 | 2,472.3 |
| Tisagenlecleucel | 279.8 | 471.9 | 971.1 | 2,143.5 |
| Brexucabtagene Autoleucel | 232.2 | 393.7 | 814.8 | 1,808.5 |
| Others | 270.2 | 452.0 | 922.8 | 2,020.3 |
By Indication
The lymphoma segment holds a dominant position in the global CAR T-cell therapy market and is poised for growth in the projected period. This growth is primarily driven by the rising incidence of non-Hodgkin lymphoma cases worldwide. CAR T-cell therapy, leveraging chimeric antigen receptor (CAR) technology, harnesses patients' immune cells to target and eliminate cancerous lymphoma cells. Engineered CAR molecules enhance immune response by recognizing specific antigens on lymphoma cell surfaces, facilitating targeted therapy. As advancements continue in CAR T-cell technology and clinical applications, the lymphoma segment remains pivotal in shaping the evolving landscape of oncological treatment options, underscoring its significant role and potential growth in the market.
The acute lymphocytic leukemia (ALL) segment is poised to experience rapid growth, driven by its challenging prognosis, particularly in cases of relapsed or refractory disease. T-cell acute lymphoblastic leukemia (T-ALL) presents fewer treatment options compared to B-cell ALL, necessitating innovative approaches like CAR T-cell therapy. Using autologous CAR T-cells in T-ALL is complicated by shared antigens with normal T-cells, leading to fratricide and potential T-cell aplasia if CAR T-cells persist. Contamination risk of the apheresis product by lymphoblasts further complicates therapy. To mitigate these challenges, various T-cell editing methods, such as protein expression blockers, CRISPR/Cas9, and base-editing, are employed. These advancements in T-cell engineering aim to enhance CAR T-cell therapy efficacy and safety, addressing critical unmet needs in treating T-ALL and driving growth in this segment of the CAR T-cell therapy market.
CAR T-Cell Therapy Market Revenue, By Indication, 2020 to 2023 (US$ Million)
| Indication | 2020 | 2021 | 2022 | 2023 |
| Lymphoma | 545.7 | 917.1 | 1,881.0 | 4,137.9 |
| Acute Lymphocytic Leukemia | 415.7 | 703.8 | 1,454.4 | 3,223.1 |
| Others | 142.0 | 239.1 | 491.6 | 1,083.6 |
By End User
The hospital segment holds the largest market share in the global CAR T-cell therapy market, driven by its role in managing patients undergoing this advanced cancer treatment. CAR T-cell therapy recipients typically undergo a risk and recovery period lasting approximately 2 to 3 months post-treatment, during which they are closely monitored for treatment response and potential side effects. Hospital admissions are common during this period to manage and mitigate complications that may arise from the therapy. This underscores hospitals' pivotal role as primary care providers and treatment centers for CAR T-cell therapy patients, supporting their comprehensive medical needs from initial treatment through recovery phases.
The cancer treatment center segment is expected to experience the highest compound annual growth rate (CAGR) during the forecast period in the global CAR T-cell therapy market. These centers play a crucial role in advancing CAR T-cell therapy through basic laboratory research, clinical trials, and epidemiological studies aimed at understanding cancer patterns, causes, and management strategies across diverse patient groups. Their involvement in multicenter clinical trials facilitates collaboration and patient enrollment from various regions, enhancing the breadth and depth of research outcomes. As pivotal hubs for oncological research and patient care, cancer treatment centers contribute significantly to shaping the future of CAR T-cell therapy, driving innovation and expanding treatment options for cancer patients globally.
CAR T-Cell Therapy Market Revenue, By End User, 2020 to 2023 (US$ Million)
| End User | 2020 | 2021 | 2022 | 2023 |
| Hospitals | 591.7 | 1,003.3 | 2,076.4 | 4,608.8 |
| Cancer Treatment Centers | 511.7 | 856.7 | 1,750.6 | 3,835.8 |
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/6000
Market Dynamics
Driver
Clinical Success and Advancements in CAR T-Cell Therapy
CAR T-cell therapy represents a pioneering approach to treating refractory blood cancers, offering a potential cure where conventional treatments have faltered. Initially developed for acute lymphoblastic leukemia (ALL), particularly in pediatric cases where intensive chemotherapy achieves high cure rates for B-cell types. Challenges persist for patients experiencing relapses post-chemotherapy or stem-cell transplant, highlighting the critical need for effective alternatives. CAR T-cell therapies address this gap by harnessing the body's immune system to target cancer cells, showcasing significant clinical successes in achieving remission and potential cures. These advancements drive optimism and market growth potential for CAR T-cell therapies, positioning them as transformative treatments in oncology.
In May 2024, Astellas and Poseida Therapeutics entered into a research collaboration and license agreement to develop novel allogeneic cell therapies in oncology.
Restraint
Side Effects and Care Requirements
CAR T-cell therapy can be highly effective against some types of hard-to-treat cancers, it also presents significant challenges that may constrain market growth. The therapy can cause serious or even life-threatening side effects, necessitating administration in specialized medical centers with trained staff. Patients require close monitoring for several weeks post-treatment to manage potential complications.
One major side effect is Cytokine Release Syndrome (CRS), where multiplying CAR T cells release large amounts of cytokines into the blood, excessively stimulating the immune system. This can lead to severe side effects, including high fever and chills, trouble breathing, severe nausea, vomiting, diarrhea, dizziness, lightheadedness, and headaches. These risks and the need for specialized care facilities limit the widespread adoption and growth of the CAR T-cell therapy market.
Opportunity
Expanding Applications of CAR-T Cell Therapy
Modern trends and recent developments in CAR-T cell therapy are increasingly focusing on diseases beyond cancer, such as autoimmune disorders and viral infections, including SARS-CoV-2. The number of available targets for CAR-T cell therapy is rapidly expanding, driven by the growing understanding of various pathologies and their fundamental molecular mechanisms. This progress is pushing the application boundaries of cellular immunotherapy far beyond oncology.
CAR-T cell technology holds great promise for treating viral infections in patients with primary immune deficiency (PID). Ongoing research in this field offers hope for many currently incurable diseases, potentially leading to novel CAR-T cell therapies and paving the way for future breakthroughs in clinical applications. These advancements create significant growth opportunities for the CAR-T cell therapy market.
Immediate Delivery Available, Get Full Access@ https://www.novaoneadvisor.com/report/checkout/6000
CAR T-Cell Therapy Market Top Key Companies:
- Johnson & Johnson Services, Inc.
- ALLOGENE THERAPEUTICS
- Lonza
- Aurora Biopharma
- Cartesian Therapeutics, Inc.
- Novartis
- Bristol-Myers Squibb company
- Gilead Sciences
- Curocell Inc
- JW Therapeutics
- In May 2024, TC BioPharm announced the execution of a second non-binding letter of intent for acquisition, targeting innovative CAR-T therapies.
- In March 2024, Mustang Bio announced its vision for expanding its CAR T-cell therapy platform into autoimmune diseases.
- In July 2024, Innovent and IASO Bio enhanced their strategic collaboration in cell therapy.
- In May 2024, Galapagos and Adaptimmune signed a clinical collaboration agreement with an option to exclusively license Adaptimmune’s TCR T-cell therapy candidate, uza-cel, in head & neck cancer and potential future solid tumor indications.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the CAR T-Cell Therapy market.
By Drug Type
- Axicabtagene Ciloleucel
- Tisagenlecleucel
- Brexucabtagene Autoleucel
- Others
- Lymphoma
- Acute Lymphocytic Leukemia
- Chronic Lymphocytic Leukemia (CLL)
- Multiple Myeloma (MM)
- Others
- Hospitals
- Cancer Treatment Centers
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
https://www.novaoneadvisor.com/report/checkout/6000
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the CAR T-Cell Therapy Market analysis from 2021 to 2033 to identify the prevailing CAR T-Cell Therapy Market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the cancer supportive care drugs industry segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global CAR T-Cell Therapy Market trends, key players, market segments, application areas, and market growth strategies.
Cell Therapy Market Size and Forecast: The global cell therapy market size was exhibited at USD 4.85 billion in 2023 and is projected to hit around USD 37.42 billion by 2033, growing at a CAGR of 22.67% during the forecast period 2024 to 2033.
Gene Therapy Market Size and Forecast : The global gene therapy market size was exhibited at USD 8.75 billion in 2023 and is projected to hit around USD 52.40 billion by 2033, growing at a CAGR of 19.6% during the forecast period 2024 to 2033.
Cell And Gene Therapy Manufacturing Market Size and Forecast: The global cell and gene therapy manufacturing market size was estimated at USD 9.95 billion in 2023 and is projected to hit around USD 106.03 billion by 2033, growing at a CAGR of 26.7% during the forecast period from 2024 to 2033.
Cell and Gene Therapy Market Size and Forecast: The global cell and gene therapy market size was estimated at USD 18.13 billion in 2023 and is projected to hit around USD 97.33 billion by 2033, growing at a CAGR of 18.3% during the forecast period from 2024 to 2033.
U.S. Cell Therapy Market Size and Forecast: The U.S. cell therapy market size was estimated at USD 2.88 billion in 2023 and is projected to hit around USD 19.67 billion by 2033, growing at a CAGR of 21.18% during the forecast period from 2024 to 2033.
U.S. Gene Therapy Market Size and Forecast: The U.S. gene therapy market size was estimated at USD 3.19 billion in 2023 and is projected to hit around USD 18.50 billion by 2033, growing at a CAGR of 19.22% during the forecast period from 2024 to 2033.
T-cell Therapy Market Size and Forecast: The global T-Cell therapy market size was exhibited at USD 3.85 billion in 2023 and is projected to hit around USD 79.62 billion by 2033, growing at a CAGR of 35.38% during the forecast period 2024 to 2033.
Cancer Immunotherapy Market Size and Forecast: The global cancer immunotherapy market size was valued at USD 126.19 billion in 2023 and is projected to surpass around USD 296.01 billion by 2033, registering a CAGR of 8.9% over the forecast period of 2024 to 2033.
Immunotherapy Drugs Market : The global immunotherapy drugs market size was valued at USD 240.19 billion in 2023 and is projected to surpass around USD 1,300.38 billion by 2033, registering a CAGR of 18.4% over the forecast period of 2024 to 2033.
Clinical Trials Market Size and Forecast: The global clinical trials market size was estimated at USD 81.90 billion in 2023 and is projected to hit around USD 153.59 billion by 2033, growing at a CAGR of 6.49% during the forecast period from 2024 to 2033.
Biologics Market Size and Forecast: The global biologics market size was estimated at USD 511.04 billion in 2023 and is projected to hit around USD 1,374.51 billion by 2033, growing at a CAGR of 10.4% during the forecast period from 2024 to 2033.
Biotechnology Market Size and Forecast: The global biotechnology market size was estimated at USD 1.54 Trillion in 2023 and is projected to hit around USD 5.68 Trillion by 2033, growing at a CAGR of 13.95% during the forecast period from 2024 to 2033.
Oncology Market Size and Forecast: The global oncology market size was estimated at USD 222.36 billion in 2023 and is projected to hit around USD 521.60 billion by 2033, growing at a CAGR of 8.9% during the forecast period from 2024 to 2033.
Cell And Gene Therapy Clinical Trials Market Size and Forecast: The global cell and gene therapy clinical trials market size reached USD 11.62 billion in 2023 and is projected to hit around USD 47.40 billion by 2033, expanding at a CAGR of 15.09% during the forecast period from 2024 to 2033.
U.S. Clinical Trials Market Size and Forecast: The U.S. clinical trials market size was valued at USD 25.81 billion in 2023 and is projected to surpass around USD 41.57 billion by 2033, registering a CAGR of 4.88% over the forecast period of 2024 to 2033.
Procure Complete Report (220+ Pages PDF with Insights, Charts, Tables, and Figures) @
https://www.novaoneadvisor.com/report/checkout/6000
About Us
Nova One Advisor is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Nova One Advisor has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defines, among different ventures present globally.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Email: sales@novaoneadvisor.com
Web: https://www.novaoneadvisor.com/