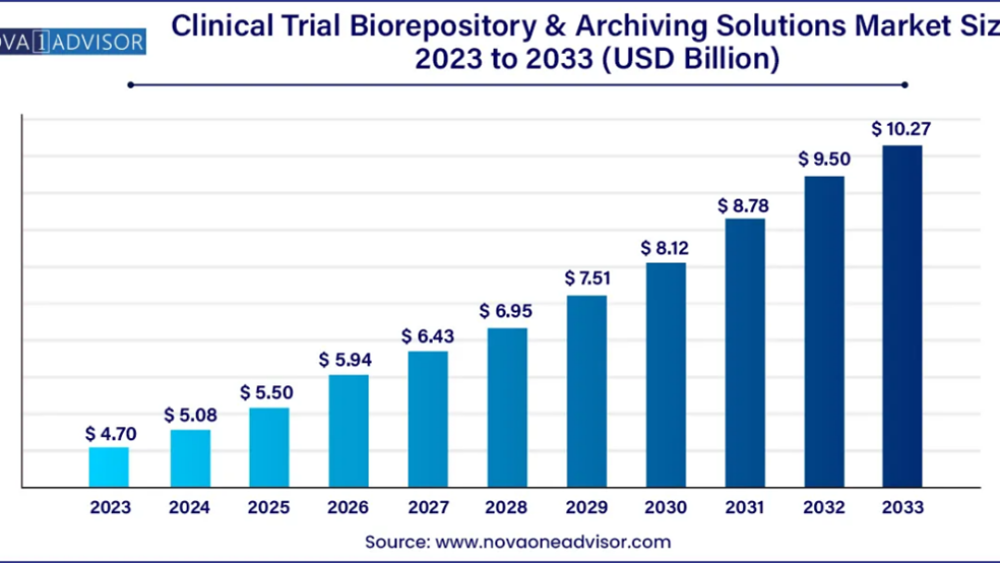
According to Nova one advisor, The global clinical trial biorepository & archiving solutions market size was estimated at USD 4.70 billion in 2023 and is projected to reach USD 10.27 billion by 2033, registering at a CAGR of 8.13% from 2024 to 2033.
Biorepositories support a wide array of research purposes, from focused medical studies targeting specific diseases, such as identifying genetic mutations, to broad population-based research. Some biorepositories are established by medical centers or organizations to collect and store biospecimens from a diverse patient population, while others are funded specifically for research studies to gather specimens from participants. This diversity in application and funding mechanisms is a significant growth driver for the clinical trial biorepository & archiving solutions market, as it underscores the critical role of biorepositories in advancing both targeted and population-based research initiatives.
Request a PDF Sample Report: https://www.novaoneadvisor.com/report/sample/7148
Key Takeaways:
The clinical trial biorepository & archiving solutions market is experiencing rapid growth due to the establishment of open-access biorepositories, which have become crucial for accelerating early research and the development of new technologies. Biorepositories are now globally diverse, with disease and population-specific biobanks addressing various health issues. Particularly, biorepositories focused on diseases prevalent in global South settings are ensuring proper ownership and attribution to the communities and scientists contributing samples, even when housed in global North geographies.
Clinical trials, involving volunteer participation to answer specific health questions, rely on biobanks for the effective management and storage of critical biospecimens, ensuring specimen integrity for robust and reproducible results. Archiving trial documents is essential for preserving knowledge for future reference. The quality of data generated through these trials significantly influences the outcomes, driving the demand for advanced biorepository and archiving solutions across pharma, biotech, diagnostic labs, and contract research organizations.
· In March 2024, Tobin Scientific acquired BioRepository Resources LLC (BRR), a leader in biological sample storage and management solutions. This strategic acquisition enhances Tobin Scientific’s capabilities in biopharmaceutical storage and expands its presence in the life sciences industry.
Buy Full Report: https://www.novaoneadvisor.com/report/checkout/7148
North America, the Largest Shareholder in the Industry
North America, particularly the United States, dominates the clinical trial biorepository & archiving solutions market. U.S. investigators enroll approximately two-thirds of the subjects compared to their counterparts in other regions, reflecting strong domestic support for government investment in medical and scientific research. Clinical trial sponsors, which include individuals, institutions, companies, and government agencies, are crucial for initiating, managing, and financing trials, though they do not conduct the research.
· The Accelerating Clinical Trials (ACT) Canada Consortium exemplifies regional efforts to enhance the efficiency and impact of randomized controlled trials, aiming to translate results into improved health outcomes both in Canada and globally. This strong infrastructure and support network contribute significantly to North America's leading position in the market.
Asia Pacific is Observed to Grow at the Fastest Rate During the Forecast Period
Asia Pacific is rapidly emerging as the fastest growing region in the global clinical trial biorepository & archiving solutions market. As of 2023, APAC accounted for 57% of Phase I trials and 49% of Phase II trials worldwide, with a significant share of Phase III trials as well. The region's dominance is attributed to its vast patient population, favorable regulatory environment, cost-effectiveness, high-quality standards, and the presence of leading clinical institutions. Notably, China's regulatory reforms have significantly accelerated drug approval processes, resulting in the highest number of new trials in the region during the assessed period. These factors collectively make APAC a preferred destination for conducting clinical trials and contribute to its rapid market growth.
Recent Breakthroughs in Clinical Trial Biorepository & Archiving Solutions Market
· In June 2024, Clinigen announced the acquisition of Ascenian, a boutique global market access consultancy. The combined entities will continue to accelerate access to life-saving treatments, delivering innovative therapies to patients faster, marking a significant milestone in Clinigen’s evolution towards becoming a premier global provider of high-value services to the pharmaceutical and biotechnology sectors.
· In August 2024, Boehringer Ingelheim and CBmed GmbH Center for Biomarker Research in Medicine (CBmed) announced a long-term strategic partnership. The collaboration aims to apply translational medicine approaches to accelerate the development of first-in-class medicines, with the goal of curing a range of cancers and transforming the lives of people with cancer.
Clinical Trial Biorepository & Archiving Solutions Market Segments
By Product, clinical segment led the market.
The clinical segment held a commanding share of the global market and is expected to sustain its dominance throughout the forecast period. Clinical research is crucial for advancing medical knowledge and improving patient outcomes by evaluating the safety and efficacy of new interventions. These trials yield valuable insights that inform treatment decisions and enhance care quality. Furthermore, clinical research drives the development of innovative medical technologies, pharmaceuticals, and treatment modalities, solidifying its central role in the market.
The preclinical segment is poised for the fastest growth during the analyzed period. Preclinical studies are essential for establishing the efficacy and safety of new chemical entities (NCEs) before human trials. To be valuable, these experiments must be rigorously designed and yield outcomes that are both relevant and translatable to human applications. Preclinical research plays a critical role in guiding the development of novel diagnostics and therapeutics, with results from animal models often determining the feasibility of subsequent human studies. This foundational research is thus crucial for advancing new medical innovations and is driving significant growth in the preclinical segment.
By Phase, Phase III segment led the market.
The Phase III segment dominates the clinical trial biorepository & archiving solutions market. Phase III studies involve extensive testing across multiple centers, with hundreds to thousands of patients who are the target population for the drug. This large-scale testing generates substantial data on the drug's safety and efficacy, reinforcing its pivotal role in the clinical trial process. The extensive data collection and management requirements associated with Phase III trials contribute to the segment's dominance in the market.
· In September 2022, Sandoz announced further progress on its biosimilar pipeline with positive results from the integrated ROSALIA Phase I/III clinical trial study for its proposed biosimilar denosumab.
The Phase II segment is projected to experience the fastest growth during the forecast period. Phase II studies are critical for evaluating the effectiveness of an experimental drug on a specific disease or condition, involving approximately 100 to 300 volunteers. This phase typically spans from several months to two years, focusing on determining the drug's efficacy and optimal dosing. The expanding role of Phase II trials in early drug development and their contribution to shaping subsequent research stages are driving significant growth in this segment.
By Services, biorepository services segment led the market.
The biorepository segment commands the largest revenue share in the market. Biorepositories play a crucial role by providing high-quality patient specimens for biomarker assessment, which is essential for personalized therapy. They offer a valuable repository of mutational data linked to clinical outcomes, aiding in the identification of clinically relevant mutations. Serving as libraries for biospecimens, biorepositories are instrumental for scientists conducting clinical or research studies, underpinning their dominance and significant revenue contribution in the market.
The archiving solutions segment is anticipated to register the fastest growth rate during the forecast period, driven by increasing medical research and clinical trials. In the medical industry, archiving plays a vital role in maintaining comprehensive patient records, enabling accurate diagnoses by tracking historical medical data. This storage solution not only facilitates easy access to medical histories for both patients and healthcare providers but also enhances the efficiency of healthcare delivery. The dual benefits of archiving providing essential information for accurate treatment and supporting patient accessibility underscore its growing importance and rapid expansion in the market.
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/7148
Some of the prominent players in the Clinical trial biorepository & archiving solutions market include:
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the global clinical trial biorepository & archiving solutions market.
Service
Nova One Advisor Group Offer Other Reports:
U.S. Clinical Trials Market : The U.S. clinical trials market size was valued at USD 25.81 billion in 2023 and is projected to surpass around USD 41.57 billion by 2033, registering a CAGR of 4.88% over the forecast period of 2024 to 2033.
Clinical Trials Market : The global clinical trials market size was estimated at USD 81.90 billion in 2023 and is projected to hit around USD 153.59 billion by 2033, growing at a CAGR of 6.49% during the forecast period from 2024 to 2033.
Biotechnology Market : The global biotechnology market size was estimated at USD 1.54 Trillion in 2023 and is projected to hit around USD 5.68 Trillion by 2033, growing at a CAGR of 13.95% during the forecast period from 2024 to 2033.
Clinical Trial Supplies Market: The clinical trial supplies market size was exhibited at USD 3.55 billion in 2023 and is projected to hit around USD 8.49 billion by 2033, growing at a CAGR of 9.11% during the forecast period 2024 to 2033.
U.S. Preclinical CRO Market: The U.S. preclinical CRO market size was valued at USD 3.19 billion in 2023 and is projected to surpass around USD 6.39 billion by 2033, registering a CAGR of 7.2% over the forecast period of 2024 to 2033.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Email: sales@novaoneadvisor.com
Web: https://www.novaoneadvisor.com/
Biorepositories support a wide array of research purposes, from focused medical studies targeting specific diseases, such as identifying genetic mutations, to broad population-based research. Some biorepositories are established by medical centers or organizations to collect and store biospecimens from a diverse patient population, while others are funded specifically for research studies to gather specimens from participants. This diversity in application and funding mechanisms is a significant growth driver for the clinical trial biorepository & archiving solutions market, as it underscores the critical role of biorepositories in advancing both targeted and population-based research initiatives.
Request a PDF Sample Report: https://www.novaoneadvisor.com/report/sample/7148
Key Takeaways:
- The North America region dominated the global industry in 2023 and accounted for the maximum share of more than 49.0% of the overall revenue.
- The U.S. clinical trial biorepository & archiving solutions market size was exhibited at USD 1.61 billion in 2023 and is projected to hit around USD 3.58 billion by 2033, growing at a CAGR of 8.3% during the forecast period 2024 to 2033.
- In terms of service, the biorepository services segment dominated the industry and accounted for the largest share of 66.6% in 2023.
- The archiving solution services segment is also expected to have significant growth over the forecast period
- The clinical products segment dominated the global industry in 2023 and accounted for the maximum share of more than 63.25% of the overall revenue.
- The phase III clinical trials segment dominated the global industry in 2023 and accounted for the largest share of more than 53.00% of the overall revenue.
- The Asia Pacific regional market is anticipated to register the fastest growth rate during the forecast period.
The clinical trial biorepository & archiving solutions market is experiencing rapid growth due to the establishment of open-access biorepositories, which have become crucial for accelerating early research and the development of new technologies. Biorepositories are now globally diverse, with disease and population-specific biobanks addressing various health issues. Particularly, biorepositories focused on diseases prevalent in global South settings are ensuring proper ownership and attribution to the communities and scientists contributing samples, even when housed in global North geographies.
Clinical trials, involving volunteer participation to answer specific health questions, rely on biobanks for the effective management and storage of critical biospecimens, ensuring specimen integrity for robust and reproducible results. Archiving trial documents is essential for preserving knowledge for future reference. The quality of data generated through these trials significantly influences the outcomes, driving the demand for advanced biorepository and archiving solutions across pharma, biotech, diagnostic labs, and contract research organizations.
· In March 2024, Tobin Scientific acquired BioRepository Resources LLC (BRR), a leader in biological sample storage and management solutions. This strategic acquisition enhances Tobin Scientific’s capabilities in biopharmaceutical storage and expands its presence in the life sciences industry.
Buy Full Report: https://www.novaoneadvisor.com/report/checkout/7148
North America, the Largest Shareholder in the Industry
North America, particularly the United States, dominates the clinical trial biorepository & archiving solutions market. U.S. investigators enroll approximately two-thirds of the subjects compared to their counterparts in other regions, reflecting strong domestic support for government investment in medical and scientific research. Clinical trial sponsors, which include individuals, institutions, companies, and government agencies, are crucial for initiating, managing, and financing trials, though they do not conduct the research.
· The Accelerating Clinical Trials (ACT) Canada Consortium exemplifies regional efforts to enhance the efficiency and impact of randomized controlled trials, aiming to translate results into improved health outcomes both in Canada and globally. This strong infrastructure and support network contribute significantly to North America's leading position in the market.
Asia Pacific is Observed to Grow at the Fastest Rate During the Forecast Period
Asia Pacific is rapidly emerging as the fastest growing region in the global clinical trial biorepository & archiving solutions market. As of 2023, APAC accounted for 57% of Phase I trials and 49% of Phase II trials worldwide, with a significant share of Phase III trials as well. The region's dominance is attributed to its vast patient population, favorable regulatory environment, cost-effectiveness, high-quality standards, and the presence of leading clinical institutions. Notably, China's regulatory reforms have significantly accelerated drug approval processes, resulting in the highest number of new trials in the region during the assessed period. These factors collectively make APAC a preferred destination for conducting clinical trials and contribute to its rapid market growth.
Recent Breakthroughs in Clinical Trial Biorepository & Archiving Solutions Market
· In June 2024, Clinigen announced the acquisition of Ascenian, a boutique global market access consultancy. The combined entities will continue to accelerate access to life-saving treatments, delivering innovative therapies to patients faster, marking a significant milestone in Clinigen’s evolution towards becoming a premier global provider of high-value services to the pharmaceutical and biotechnology sectors.
· In August 2024, Boehringer Ingelheim and CBmed GmbH Center for Biomarker Research in Medicine (CBmed) announced a long-term strategic partnership. The collaboration aims to apply translational medicine approaches to accelerate the development of first-in-class medicines, with the goal of curing a range of cancers and transforming the lives of people with cancer.
Clinical Trial Biorepository & Archiving Solutions Market Segments
By Product, clinical segment led the market.
The clinical segment held a commanding share of the global market and is expected to sustain its dominance throughout the forecast period. Clinical research is crucial for advancing medical knowledge and improving patient outcomes by evaluating the safety and efficacy of new interventions. These trials yield valuable insights that inform treatment decisions and enhance care quality. Furthermore, clinical research drives the development of innovative medical technologies, pharmaceuticals, and treatment modalities, solidifying its central role in the market.
The preclinical segment is poised for the fastest growth during the analyzed period. Preclinical studies are essential for establishing the efficacy and safety of new chemical entities (NCEs) before human trials. To be valuable, these experiments must be rigorously designed and yield outcomes that are both relevant and translatable to human applications. Preclinical research plays a critical role in guiding the development of novel diagnostics and therapeutics, with results from animal models often determining the feasibility of subsequent human studies. This foundational research is thus crucial for advancing new medical innovations and is driving significant growth in the preclinical segment.
By Phase, Phase III segment led the market.
The Phase III segment dominates the clinical trial biorepository & archiving solutions market. Phase III studies involve extensive testing across multiple centers, with hundreds to thousands of patients who are the target population for the drug. This large-scale testing generates substantial data on the drug's safety and efficacy, reinforcing its pivotal role in the clinical trial process. The extensive data collection and management requirements associated with Phase III trials contribute to the segment's dominance in the market.
· In September 2022, Sandoz announced further progress on its biosimilar pipeline with positive results from the integrated ROSALIA Phase I/III clinical trial study for its proposed biosimilar denosumab.
The Phase II segment is projected to experience the fastest growth during the forecast period. Phase II studies are critical for evaluating the effectiveness of an experimental drug on a specific disease or condition, involving approximately 100 to 300 volunteers. This phase typically spans from several months to two years, focusing on determining the drug's efficacy and optimal dosing. The expanding role of Phase II trials in early drug development and their contribution to shaping subsequent research stages are driving significant growth in this segment.
By Services, biorepository services segment led the market.
The biorepository segment commands the largest revenue share in the market. Biorepositories play a crucial role by providing high-quality patient specimens for biomarker assessment, which is essential for personalized therapy. They offer a valuable repository of mutational data linked to clinical outcomes, aiding in the identification of clinically relevant mutations. Serving as libraries for biospecimens, biorepositories are instrumental for scientists conducting clinical or research studies, underpinning their dominance and significant revenue contribution in the market.
The archiving solutions segment is anticipated to register the fastest growth rate during the forecast period, driven by increasing medical research and clinical trials. In the medical industry, archiving plays a vital role in maintaining comprehensive patient records, enabling accurate diagnoses by tracking historical medical data. This storage solution not only facilitates easy access to medical histories for both patients and healthcare providers but also enhances the efficiency of healthcare delivery. The dual benefits of archiving providing essential information for accurate treatment and supporting patient accessibility underscore its growing importance and rapid expansion in the market.
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/7148
Some of the prominent players in the Clinical trial biorepository & archiving solutions market include:
- Azenta U.S., Inc.
- Thermo Fisher Scientific Inc. (Patheon)
- Precision for Medicine, Inc.
- Medpace
- LabCorp Drug Development
- ATCC
- Q2 Solutions
- Labconnect
- Charles River Laboratories
- Cell&Co
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the global clinical trial biorepository & archiving solutions market.
Service
- Biorepository Services
- Warehousing & Storage
- Transportation
- Sample Processing
- Others
- Archiving Solution Services
- Database Indexing & Management
- Scanning & Destruction
- Preclinical Products
- Clinical Products
- Human Tissue
- Organs
- Stem Cells
- Other Biospecimens
- Phase I
- Phase II
- Phase III
- Phase IV
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
Nova One Advisor Group Offer Other Reports:
U.S. Clinical Trials Market : The U.S. clinical trials market size was valued at USD 25.81 billion in 2023 and is projected to surpass around USD 41.57 billion by 2033, registering a CAGR of 4.88% over the forecast period of 2024 to 2033.
Clinical Trials Market : The global clinical trials market size was estimated at USD 81.90 billion in 2023 and is projected to hit around USD 153.59 billion by 2033, growing at a CAGR of 6.49% during the forecast period from 2024 to 2033.
Biotechnology Market : The global biotechnology market size was estimated at USD 1.54 Trillion in 2023 and is projected to hit around USD 5.68 Trillion by 2033, growing at a CAGR of 13.95% during the forecast period from 2024 to 2033.
Clinical Trial Supplies Market: The clinical trial supplies market size was exhibited at USD 3.55 billion in 2023 and is projected to hit around USD 8.49 billion by 2033, growing at a CAGR of 9.11% during the forecast period 2024 to 2033.
U.S. Preclinical CRO Market: The U.S. preclinical CRO market size was valued at USD 3.19 billion in 2023 and is projected to surpass around USD 6.39 billion by 2033, registering a CAGR of 7.2% over the forecast period of 2024 to 2033.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Email: sales@novaoneadvisor.com
Web: https://www.novaoneadvisor.com/