Connecticut
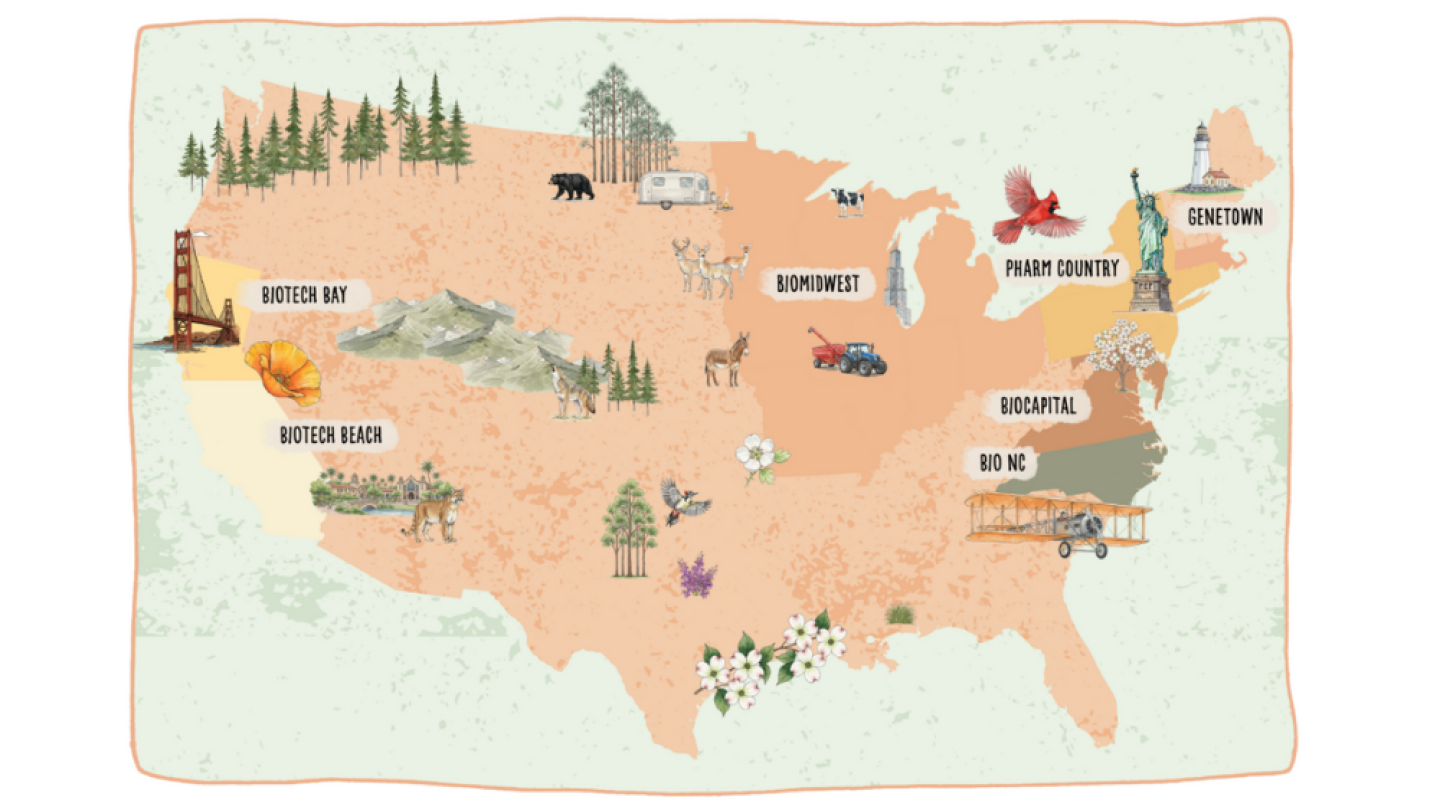
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
In 2025, made or projected biopharma workforce cuts affected about 42,700 employees, according to BioSpace tallies. BioSpace takes a deep dive into which companies and locations were impacted and speaks to experts about what to expect ahead—and why.
This year, Novo Nordisk and Merck announced significant layoffs, with Novo planning to axe about 9,000 employees and Merck projecting it could let go of roughly 6,000. Meanwhile, Bayer, Bristol Myers Squibb, Novartis and Pfizer have also made noteworthy cuts.
Over 80% of those living outside of biopharma’s biggest hubs, like San Diego or Boston, have a tough time finding work, according to a BioSpace LinkedIn poll. Biopharma professionals in Oregon and Connecticut and a BioSpace recruitment manager share their insights on this issue.
BioSpace has revealed its 2025 Hotbed Maps, showcasing nine regional hot spots for life sciences activity.
The FDA previously refused to review Biohaven’s candidate in the indication due to a failed late-stage trial. However, the company is now planning to file an NDA in the fourth quarter of 2024.
Thursday’s announcement comes after the insurance giant last week said it would remove AbbVie’s Humira from its major formularies starting in 2025, replacing the blockbuster with more affordable biosimilars.
The Connecticut-based biotech, which emerged from stealth last year, has secured $202 million to date as it looks to move two assets targeting prostate and breast cancer into the clinic.
A federal judge ruled last week that the U.S. government can use its economic standing as a bulk purchaser to negotiate for better deals, handing Boehringer Ingelheim a loss in its legal challenge to the Inflation Reduction Act.
PRESS RELEASES