Cyclerion is a clinical-stage biopharmaceutical company developing treatments for serious and orphan diseases affecting the central nervous system (CNS).
Cheryl Gault, Head of Strategy & Corporate Development at Cyclerion (left) and Chris Winrow, Ph.D., Senior Director, Clinical Development, and Neuroscience Program Lead at Cyclerion (right). Photos courtesy of Cyclerion.
Cyclerion Therapeutics Inc. announced top-line results from its STRONG-SCD study of Olinciguat, which did not show adequate activity in patients with sickle cell disease (SCD) to support further internal development.
Sickle cell disease is a group of blood disorders, the most common of which is sickle cell anemia. It is usually hereditary and most prevalent in the African American population. Symptoms begin as early as 5 to 6 months, and include pain, frequent infections and swelling in the hands and feet.
The Phase II study included 70 participants across several dose levels, and was designed to evaluate the safety, tolerability and pharmacokinetics of Olinciguat. While well-tolerated, the necessary impact on daily symptoms and biomarkers was not present.
“No one is more disappointed than we are at the results we announced this morning. We very much hoped to be able to be part of the solution in the sickle cell space. These are patients that have tremendous unmet needs, and even though there are a few new drugs that have recently been approved, they certainly need more, and we very much hoped to be a part of that,” Cheryl Gault, Head of Strategy & Corporate Development at Cyclerion, told BioSpace in an interview.
The company is still hopeful that information gleaned from the study could provide clues to understanding potential biomarker signals such as inflammation.
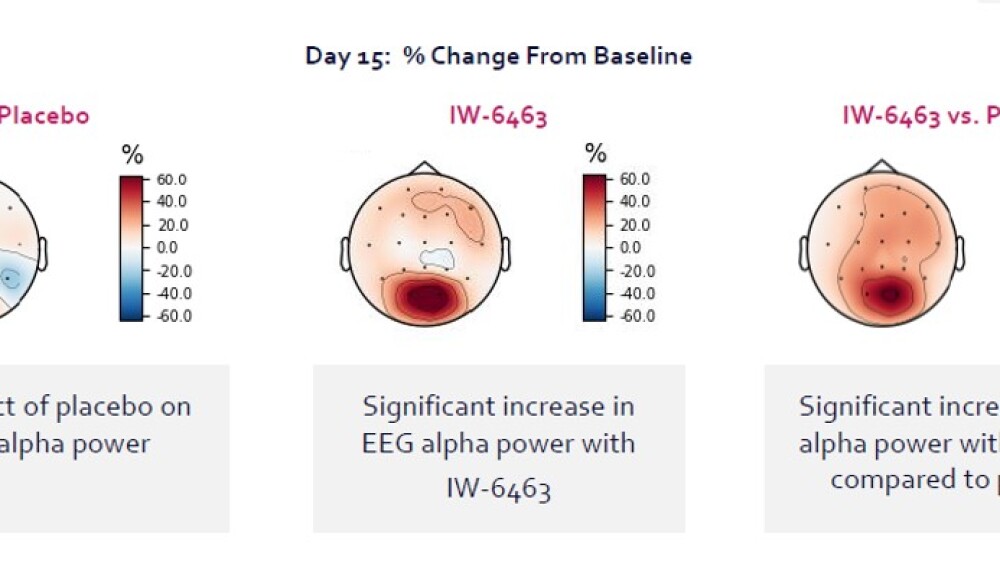
Offsetting the disappointing news from the Olinciguat study was the promising data gleaned from a Phase I translational pharmacology study of the company’s lead clinical program, IW-6463.
IW-6463 is the first soluble guanylate cyclase (sGC) stimulator in clinical development for CNS disorders, and is intended to treat mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) and Alzheimer’s Disease with vascular pathology (ADv).
There is currently no approved treatment for MELAS, a rare genetic disorder characterized by dementia and lactic acid build-up in the bloodstream.
The mechanism in IW-6463 works by stimulating the sGC, a signaling enzyme that responds to the presence of nitric oxide (NO) – a fundamental neurotransmitter – to enhance the body’s natural ability to produce cyclic guanosine monophosphate (Cgmp). Disruption of the NO-sGC-Cgmp pathway is thought to play a pivotal role in the development of neurodegenerative diseases.
“We actually saw some really exciting results where what’s called alpha power was affected significantly in these elderly individuals. Alpha power goes down with age, it also goes down with dementia and cognitive impairment, so this is, for us, a really exciting observation in Phase I, to see healthy volunteers who were actually able to effect this,” said Chris Winrow, Ph.D., Senior Director, Clinical Development, and Neuroscience Program Lead at Cyclerion.
Winrow noted that they also saw positive effects on objective saccadic eye movement, which is related to attention and cognitive processing and decreases with age and dementia.
“We’re really excited because we saw the performance in this saccadic eye movement was actually improved in the elderly volunteers. So again, these are all things you can look at in moving forward into our additional studies,” Winrow said.
Cyclerion is a clinical-stage biopharmaceutical company developing treatments for serious and orphan diseases affecting the central nervous system (CNS).
Diseases like Alzheimer’s and other dementias have emerged as one of the most critical healthcare concerns, as the number of people 65 or older is projected to hit nearly 1.5 billion by 2050.