In this deep dive, BioSpace takes a closer look at the drug price crisis in the U.S. As President Joe Biden and former President Donald Trump gear up for a rematch in the 2024 election, we explore how federal reforms to lower costs could be leveraged on the campaign trail.
In this special edition of BioPharm Executive, BioSpace takes a closer look at the drug price crisis in the U.S. As President Joe Biden and former President Donald Trump gear up for a rematch in the 2024 election, we explore how federal reforms to lower costs could be leveraged on the campaign trail.
What’s Happening
Drug prices in the U.S. are, on average, 2.78 times higher than in the other 32 Organization for Economic Cooperation and Development (OECD) countries, according to a 2024 report from RAND. Insulin in particular is nearly 10 times more expensive in the U.S. than in other industrialized nations.
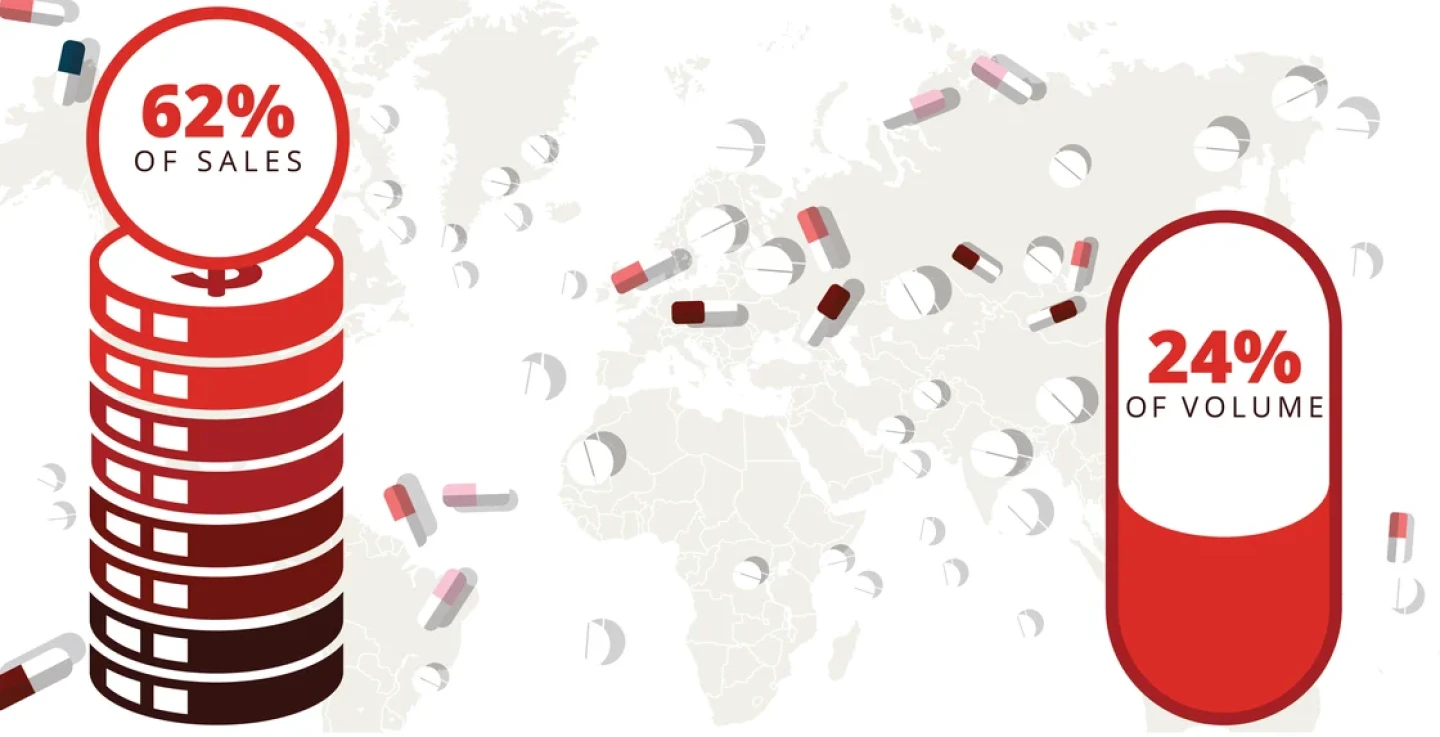
The U.S. accounted for a majority of sales across nearly three dozen OECD nations ($989 billion in 2022), but just a quarter of the volume.
Source: RAND
Biden Eyes IRA Expansion
The Inflation Reduction Act (IRA), which Biden signed into law in 2022, gives the Department of Health and Human Services (HHS) authority to negotiate the price of as many as 20 single-source prescription drugs annually under Medicare starting this year. On Feb. 1, the Centers for Medicare & Medicaid Services (CMS) sent its initial offers on the first 10 drugs selected for price negotiations, to which the companies had 30 days to respond. Negotiated rates will be announced by Sept. 1, to take effect in January 2026. Big Pharma and allies have filed lawsuits across the country to try to block the program, but they have had no success to date, losing most recently in Arkansas.
The Congressional Budget Office estimated that the Drug Price Negotiation Program will save taxpayers nearly $95 billion between 2026 and 2031. During his State of the Union address March 7, Biden called for expanding the nascent program to include 50 drugs per year, potentially boosting those savings. Biden also said he’d push for extending the $2,000 annual cap on out-of-pocket prescription drug spending beyond Medicare to all privately insured Americans.
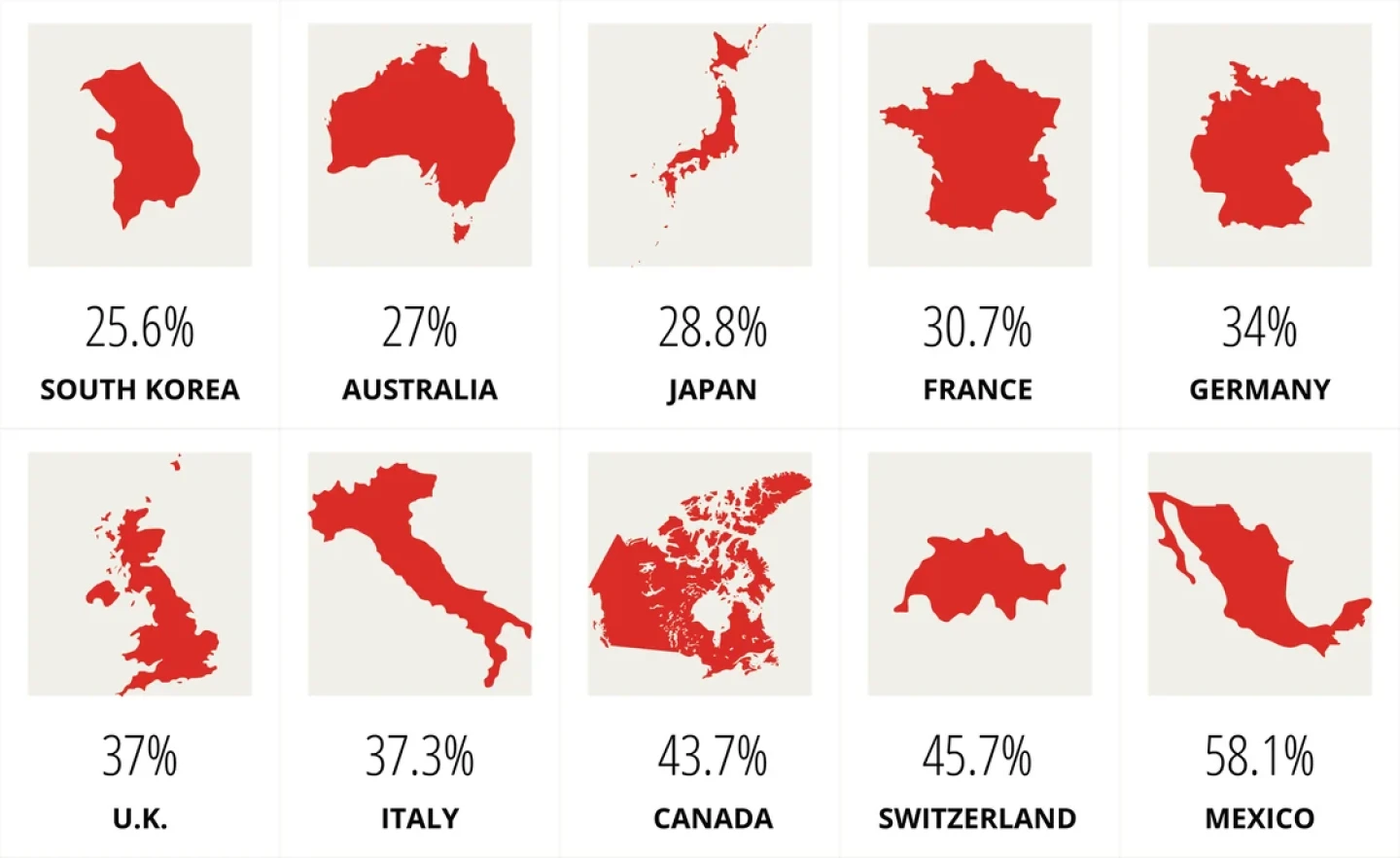
Many countries enjoy significant discounts compared to what U.S. consumers are spending on prescription medicines, largely due to single-payer health systems with tighter regulation.
Percent of US Cost
Source: RAND
Trump’s Legacy Touched on Imports and Biosimilars
The IRA is just one of a handful of federal efforts to curb drug prices in America. Drug importation, particularly from Canada, is another possible avenue to cheaper medications. In January, the FDA approved a two-year plan for Florida to import prescription drugs from north of the border. The approval came under Section 804 of the Federal Food, Drug and Cosmetic Act (FFDCA), a strategy actually championed by the Trump administration.
The Trump administration also advocated for faster approval of biosimilars to compete with high-dollar biologics like AbbVie’s blockbuster Humira. While Trump has been less vocal on the issue of drug pricing than in previous election years, he has made clear his intention to issue an executive order forcing Big Pharma to reduce prices “very substantially for American patients.” Specifically, the U.S. government “will tell Big Pharma that we will only pay the best price they offer to foreign nations,” Trump stated in a campaign video.
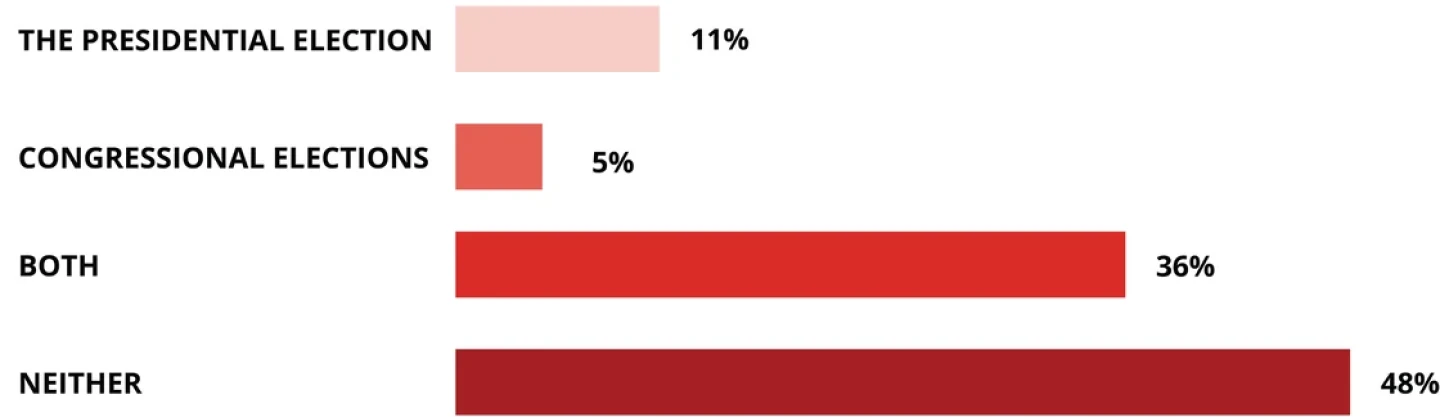
Will proposed fixes for high drug pricing in the U.S. affect your vote in any of the 2024 elections?
Source: BioSpace LinkedIn poll, March 2024
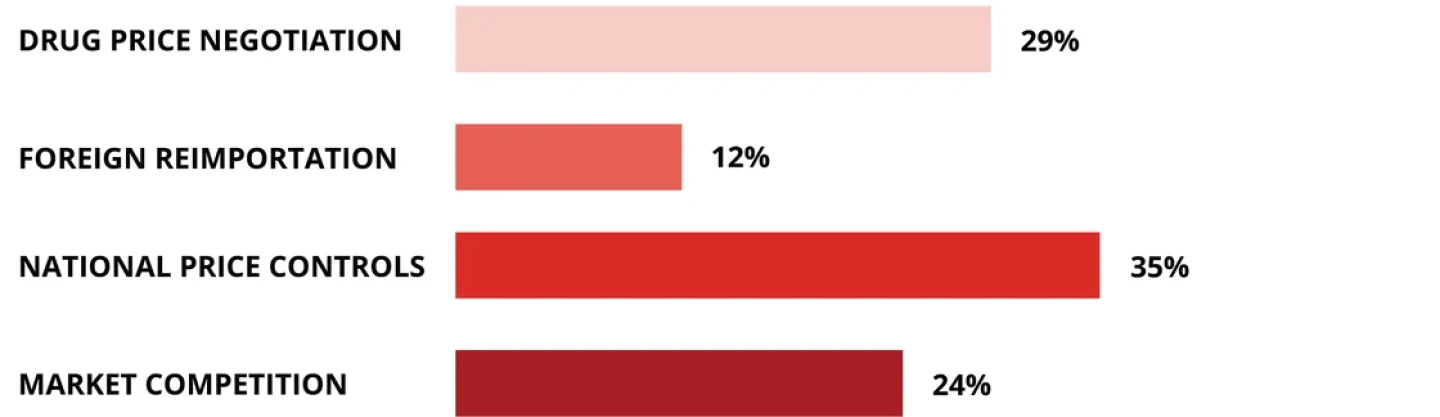
RAND released a study in February showing that prescription drug prices are 2.78 times higher in the U.S. than in 32 other OECD countries. How would you like to see this problem addressed?
Source: BioSpace LinkedIn poll, March 2024
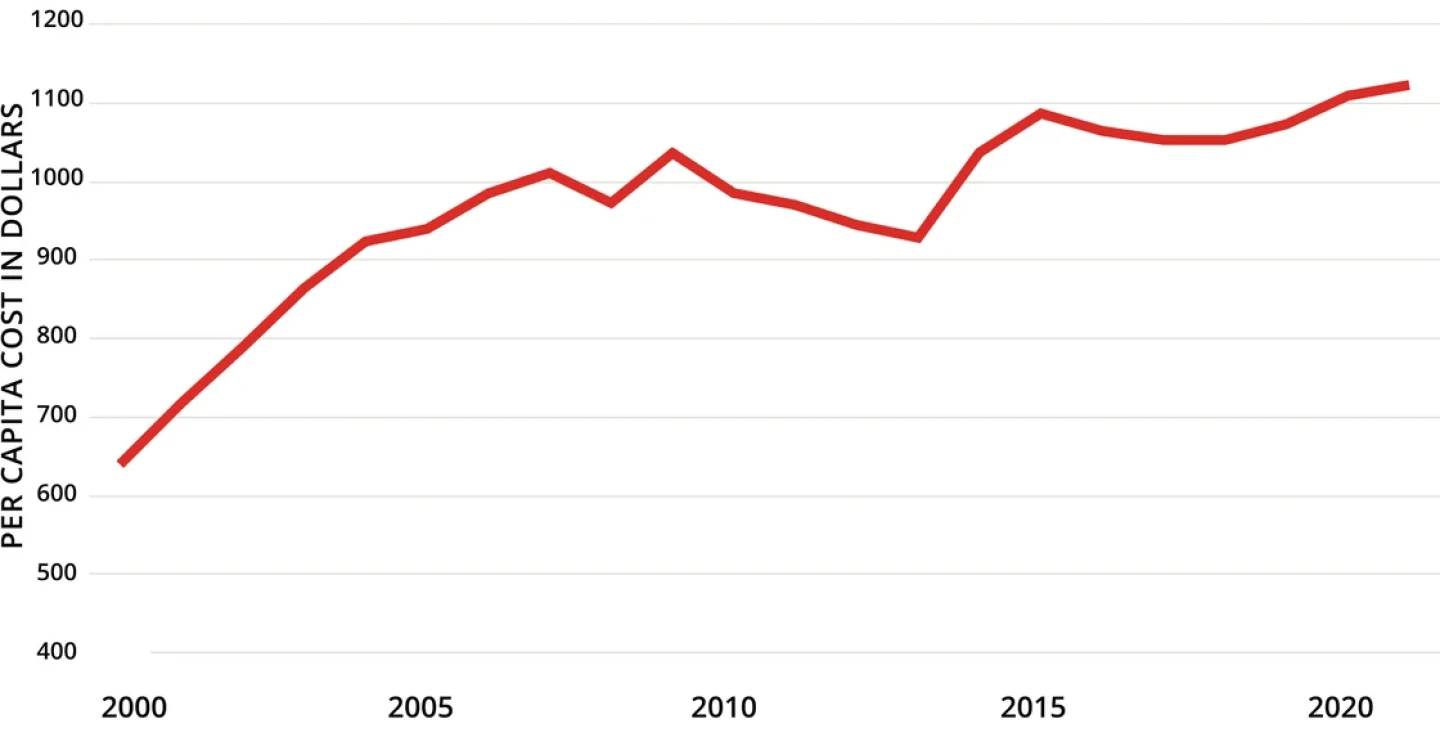
New Sticker Shock
Despite the recent changes and new proposals, drug prices in the U.S. continue to climb.
Spending on Prescription Drugs in US
Note: Data depicted are inflation-adjusted 2021 dollars.
Source: Kaiser Family Foundation analysis of National Health Expenditures Accounts
While price controls and a higher reliance on older drugs keep costs in check in other countries, another factor that could continue to inflate U.S. prices is the flurry of unprecedentedly expensive therapies hitting the market. Since 2019, the FDA has approved more than half a dozen single treatments that carry a list price of at least $2 million. And the trend continues with this month’s approval of Orchard Therapeutics’ Lenmeldy, a novel gene therapy that will cost $4.25 million, making it the most expensive treatment ever.
$4.25 million Orchard Therapeutics’ Lenmeldy for pediatric metachromatic leukodystrophy (approved 2024)
$3.5 million CSL Behring’s Hemgenix for hemophilia B (2022)
$3.2 million Sarepta Therapeutics’ Elevidys for Duchenne muscular dystrophy (2023)
$3.1 million bluebird bio’s Lyfgenia for sickle cell disease (2023)
$3 million bluebird bio’s Skysona for cerebral adrenoleukodystrophy (2022)
$2.8 million bluebird bio’s Zynteglo for ß-thalassemia (2022)
$2.2 million Vertex Pharmaceuticals’ Casgevy for sickle cell disease (2023)
$2.1 million Novartis’ Zolgensma for spinal muscular atrophy (2019)
This article was originally published as a Special Edition of Biopharm Executive, BioSpace‘s weekly newsletter covering market insights and trending stories for biopharma leaders.
For more insights like the above, subscribe today!