In this deep dive BioSpace analyzes the neuropsychedelic therapeutics pipeline, which grabbed headlines in February when the FDA accepted the New Drug Application for Lykos Therapeutics’ MDMA capsules for PTSD.
In this deep dive, BioSpace analyzes the neuropsychedelic therapeutics pipeline, which grabbed headlines in February when the FDA accepted the New Drug Application for Lykos Therapeutics’ midomafetamine (MDMA) capsules for post-traumatic stress disorder (PTSD). With an action date set for this summer, Lykos’ therapy is the first psychedelic drug for neuropsychiatric diseases up for regulatory approval in the U.S. (Johnson & Johnson does not consider Spravato, its ketamine derivative approved in 2019 as a nasal spray for depression, to be a classic psychedelic.)
Lykos could soon be joined by several other contenders. Modalities such as LSD, psilocybin and ketamine have shown promise in depression and anxiety, substance use disorders and other mental health indications, and more than three dozen companies have psychedelics-based therapies in clinical trials right now.
Where Will Psychedelic Therapies Break Through First?
Source: BioSpace LinkedIn poll, April 2024
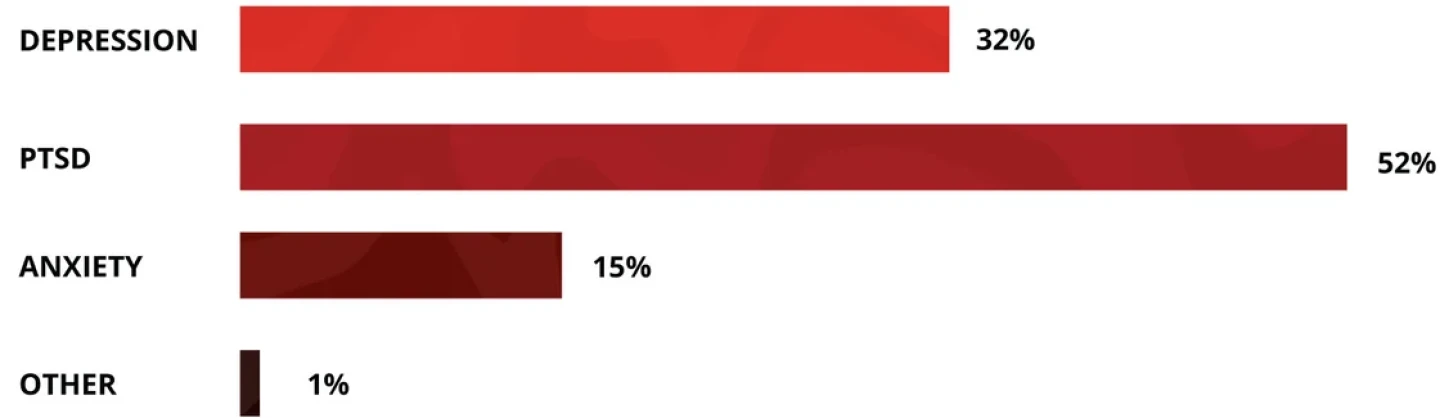
In addition to PTSD, psychedelic therapies have recently shown promise in treating various types of depression and anxiety, bipolar disorder, substance use disorders and other neuropsychiatric illnesses.
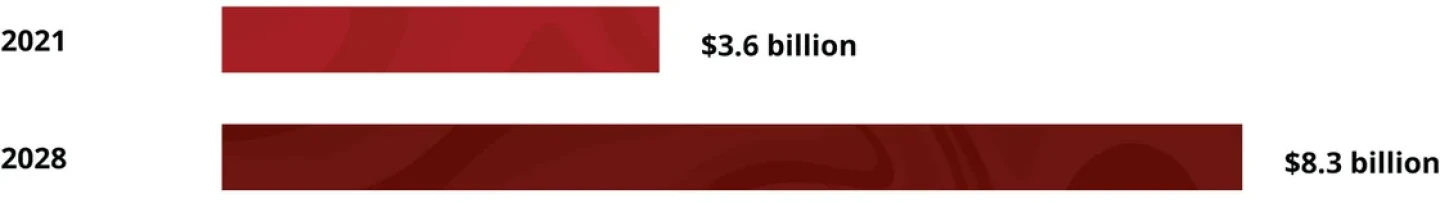
The Potential Psychedelics Market
The psychedelic therapeutics market is growing steadily. Valued at $3.6 billion in 2021, it is expected to reach $8.3 billion by 2028. Of the drugs in development, a whopping 85% are intended for neuropsychiatric diseases. Companies in this space are looking to take home a piece of the overall psychiatric treatment market, which could hit $58.91 billion by 2031.
The Psychedelics Pipeline
While Lykos is leading the charge as the only company with an asset under regulatory review, two more psychedelic therapies, from COMPASS Pathways and Awakn Life Sciences, are in Phase III, while many others are in earlier stages of R&D.
Note: This graphic was drawn from data collated by Psychedelic Alpha, is up to date as of December 2023, and does not represent all companies in Phase I and II.
Awaiting Regulatory Decision
Lykos (formerly MAPS Public Benefit Corporation) made U.S. regulatory history in December 2023 when it announced it had submitted a New Drug Application to the FDA for its MDMA capsules for PTSD. Lykos contends that its primary Phase III trial and earlier studies demonstrate that MDMA, when used in combination with psychotherapy and other supportive services provided by a qualified healthcare provider, helps patients with moderate to severe PTSD with greater efficacy than traditional treatments. The FDA has set a PDUFA date of August 11, 2024, and will hold an advisory committee meeting on June 4 to discuss the therapy.
Phase III
- COMPASS’s psilocybin-based treatment, COMP360, is being developed for treatment-resistant depression. Results from the company’s Phase IIb trial, published in the New England Journal of Medicine in November 2022, showed “a highly statistically significant reduction in depressive symptoms after three weeks” following treatment with a single dose of COMP360 in combination with psychological support, with the response lasting up to 12 weeks, according to COMPASS. A Phase III trial is underway, with a primary completion date of May 2025.
- Meanwhile, Toronto-based Awakn Life Sciences has been granted regulatory and ethical clearance for a Phase III trial in the U.K. for AWKN-001, an s-ketamine-based therapy to be used in conjunction with cognitive behavioral therapy for severe alcohol use disorder. In Phase II, treatment with the regimen led to an average of 86% abstinence over the six months following treatment vs. 2% prior to the trial, Awakn reported. Enrollment in the trial is due to begin “imminently,” Anthony Tennyson, co-founder and CEO of Awakn, told BioSpace.
Phase I and II
- The mid-stage pipeline is much more ample, with more than 30 psychedelic therapies targeting neuropsychiatric disorders in Phase II trials. Notably, more than half of these are for depression.
- There are also more than a dozen additional therapeutic candidates making their way through Phase I trials for neuropsychiatric indications, including several for substance use disorders.
Trends in Neuropsychedelic Therapeutics
Note: Data taken from Psychedelic Alpha
Treatments for depression dominate the clinical-stage pipeline, with 23 candidates in development for various depressive disorders. The second most investigated condition is substance use disorder, including alcohol use disorder. There are six investigative candidates apiece for anxiety and PTSD.
Note: Data taken from Psychedelic Alpha
Two psychedelic substances are most prevalent across the developmental pipeline: psilocybin, the active ingredient in “magic” mushrooms, and DMT, a plant-based compound used for millennia by indigenous cultures. Additional psychedelics in development include LSD, MDMA and ketamine. Most are taken under the supervision of a therapist, but some ketamine-based treatments are being developed as at-home options.
Do you think these therapies will become a staple of neuropsychiatric care?
Source: BioSpace LinkedIn poll, April 2024
This article was originally published as a Special Edition of ClinicaSpace, BioSpace‘s weekly newsletter covering biopharma research and clinical trials. You can subscribe here.