Duchenne Muscular Dystrophy Drugs Market Outlook 2024-2032:
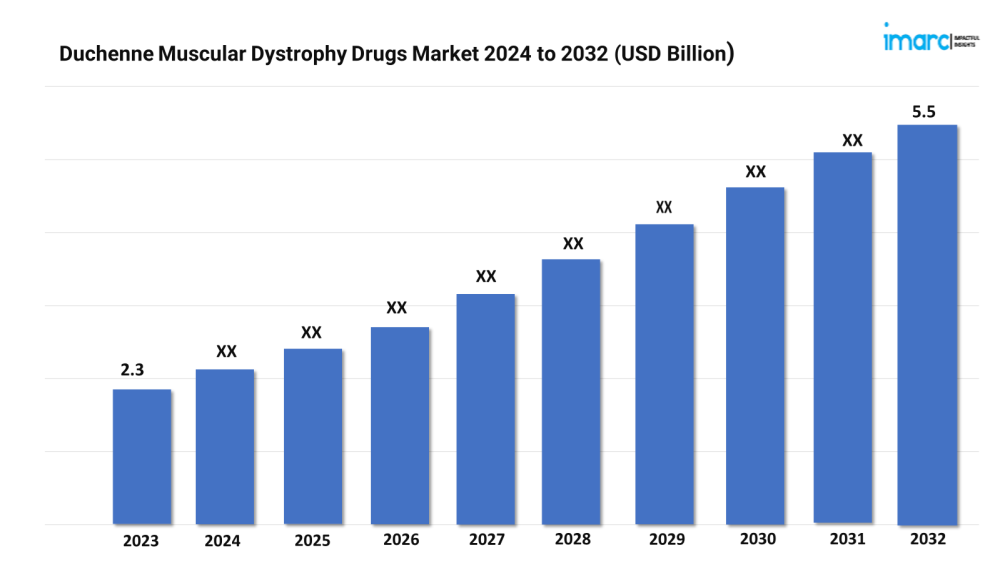
The duchenne muscular dystrophy drugs market size reached a value of USD 2.3 Billion in 2023. Looking forward, the market is expected to reach USD 5.5 Billion by 2032, exhibiting a growth rate (CAGR) of 9.96% during 2024-2032.
The market is driven by ongoing advancements and innovations in treatment surgeries, and the development of exon-skipping therapies, such as eteplirsen and golodirsen, which target specific genetic mutations associated with DMD. Additionally, there is a growing emphasis on combination therapies and personalized medicine approaches in the DMD market.
Advancements in Exon-Skipping Therapies: Driving the Duchenne Muscular Dystrophy Drugs Market
Exon-skipping therapies have emerged as a transformative approach in the treatment of Duchenne muscular dystrophy (DMD), aiming to address the underlying genetic cause of the disease. DMD is caused by mutations in the dystrophin gene, resulting in the absence or dysfunction of the dystrophin protein essential for muscle function. Exon-skipping therapies work by modulating RNA splicing to skip specific exons in the dystrophin gene transcript, enabling the production of a shorter but functional dystrophin protein fragment. Moreover, the approval of eteplirsen (Exondys 51) and golodirsen (Vyondys 53) by the U.S. Food and Drug Administration (FDA) marked significant milestones in DMD treatment. Eteplirsen targets exon 51 mutations, while golodirsen targets exon 53 mutations, collectively covering approximately 30% of DMD patients who have amenable mutations. These therapies have shown the ability to increase dystrophin expression in muscle fibers, potentially improving muscle function and slowing disease progression.
Request a PDF Sample Report: https://www.imarcgroup.com/duchenne-muscular-dystrophy-drugs-market/requestsample
Additionally, ongoing research focuses on expanding the applicability of exon-skipping therapies to include additional exons and developing new compounds with improved efficacy and delivery mechanisms. Advances in RNA-targeting technologies, such as antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), continue to enhance the specificity and efficiency of exon-skipping therapies. Moreover, efforts are underway to optimize dosing regimens and address challenges such as immune responses and delivery to target tissues. Apart from this, clinical trials are exploring the long-term safety and efficacy of exon-skipping therapies, aiming to demonstrate sustained benefits in delaying disease progression and preserving muscle function over extended periods. The evolution of these therapies represents a significant shift towards personalized medicine in DMD, tailoring treatments based on individual genetic profiles and mutations. As research progresses, exon-skipping therapies hold promise in transforming the management of DMD by addressing the root cause of the disease and potentially offering disease-modifying effects.
Gene Therapy Innovations: Contributing to Market Expansion
Gene therapy has emerged as a promising frontier in the treatment of Duchenne muscular dystrophy (DMD), offering the potential to provide a one-time, curative treatment by replacing or repairing defective genes responsible for the disease. DMD is characterized by mutations in the dystrophin gene, resulting in the absence or deficiency of dystrophin protein in muscle cells. Gene therapy approaches aim to deliver functional copies of the dystrophin gene directly to affected muscle tissues, restoring dystrophin production and potentially halting disease progression. Moreover, several gene therapy strategies are under investigation, with adeno-associated virus (AAV) vectors being commonly used to deliver therapeutic genes into muscle cells. These vectors are engineered to be safe and effective in transferring genetic material, with ongoing efforts focused on optimizing vector design, enhancing gene expression levels, and minimizing immune responses. Furthermore, recent clinical trials and preclinical studies have shown promising results for gene therapy in DMD. For example, early-stage trials have demonstrated increased dystrophin production and improvements in muscle strength and function in treated patients.
In addition to this, advances in genome editing technologies, such as CRISPR-Cas9, offer additional opportunities to precisely edit the dystrophin gene within muscle cells, potentially correcting specific mutations responsible for DMD. Challenges in gene therapy for DMD include optimizing delivery methods to ensure widespread distribution of therapeutic genes throughout muscles, minimizing immune responses to viral vectors, and achieving long-term durability of treatment effects. Research efforts are focused on addressing these challenges through innovative vector engineering, immune modulation strategies, and advancements in genome editing techniques. Besides this, the evolution of gene therapy in DMD represents a paradigm shift towards curative treatments that could transform the lives of patients and families affected by the disease. Continued research and clinical development are essential to further refine gene therapy approaches, expand treatment options, and ultimately provide effective therapies for all individuals living with DMD.
Emerging Combination Therapies:
Combination therapies are emerging as a promising strategy in the Duchenne muscular dystrophy (DMD) drugs market, aiming to synergistically target multiple disease pathways and enhance therapeutic outcomes. DMD is a complex genetic disorder characterized by progressive muscle degeneration and systemic complications, necessitating a multifaceted treatment approach. Moreover, current research explores combinations of exon-skipping therapies with supportive care interventions, such as corticosteroids, to improve muscle function and quality of life for patients. Corticosteroids, such as prednisone and deflazacort, have been the standard of care for managing DMD by reducing inflammation and delaying disease progression. When combined with exon-skipping therapies, corticosteroids may provide complementary benefits in preserving muscle strength and function.
Another area of interest is the combination of exon-skipping therapies with emerging modalities like gene editing technologies. Gene editing tools, such as CRISPR-Cas9, offer the potential to precisely edit the dystrophin gene within muscle cells, correcting specific mutations and enhancing dystrophin production. Preclinical studies have shown promise in improving muscle function and delaying disease progression in animal models of DMD, highlighting the potential synergies of combining exon-skipping with gene editing therapies. Furthermore, researchers are exploring combinations of pharmacological agents targeting different aspects of DMD pathology, such as inflammation, fibrosis, and mitochondrial dysfunction. These approaches aim to provide comprehensive disease management and improve outcomes for patients with DMD by addressing multiple disease mechanisms simultaneously. Besides this, the development of combination therapies in DMD is driven by a deeper understanding of disease complexity and the need for integrated treatment strategies. Clinical trials are underway to evaluate the safety, efficacy, and synergistic effects of these combinations, optimize treatment regimens, and improve long-term outcomes for individuals living with DMD.
Buy Full Report: https://www.imarcgroup.com/checkout?id=6715&method=502
Leading Companies in the Duchenne Muscular Dystrophy Drugs Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Duchenne muscular dystrophy drugs market, several leading companies are at the forefront for their innovative therapies and significant contributions to the field. Some of the major players include Sarepta Therapeutics Inc., Santhera Pharmaceuticals, and PTC Therapeutics Inc. These companies are actively involved in research and development to introduce new treatments and improve existing therapies, significantly impacting the lives of patients with Duchenne muscular dystrophy.
Sarepta is a significant player in the DMD and is known for its focus on genetic therapies. In June 2024, the FDA expanded the approval of Sarepta Therapeutics' gene therapy, Elevidys (delandistrogene moxeparvovec-rokl), for treating Duchenne muscular dystrophy (DMD) to include children aged 4 and above. This approval now covers both ambulatory and non-ambulatory patients with a confirmed mutation in the DMD gene.
Santhera is involved in the development of vamorolone, a novel steroid alternative, which has been launched in collaboration with Catalyst Pharmaceuticals in the United States. Also, in February 2024, Santhera published full results from its Phase 3 VISION-DMD trial, confirming the efficacy and safety of vamorolone over 48 weeks in boys with DMD. Higher doses of vamorolone (6 mg/kg/day) showed significant improvements in motor outcomes compared to lower doses. The drug also demonstrated a favorable safety profile with fewer adverse events compared to prednisone, including better growth trajectories and weight management.
Apart from this, PTC Therapeutics Inc. focuses on small molecule drugs that target genetic disorders, including DMD. In July 2024, PTC Therapeutics announced plans to submit a new drug application to the FDA for ataluren (Translarna) as a treatment for DMD caused by nonsense mutations. This follows previous conditional approval in Europe and recent discussions with the FDA to address earlier concerns regarding evidence for the drug's efficacy.
Request for customization: https://www.imarcgroup.com/request?type=report&id=6715&flag=E
Regional Analysis:
The major markets for Duchenne muscular dystrophy drugs include North America, Asia Pacific, Europe, Latin America, and the Middle East and Africa. According to projections by IMARC, North America accounted for the largest market share. This can be attributed to the rising investment in research and development to create innovative treatments for DMD, surging emphasis on personalized medicines, and collaborations between pharmaceutical companies.
Moreover, In March 2024, the FDA approved Duvyzat (givinostat), an oral histone deacetylase (HDAC) inhibitor for treating DMD in patients aged six and older. This approval followed a priority review and was based on positive results from a Phase 3 clinical trial, which demonstrated that Duvyzat significantly slowed the decline in muscle function. The most common side effects include diarrhea, abdominal pain, reduced platelet counts, and increased triglycerides.
Besides this, in June 2024, Sarepta Therapeutics' Elevidys (delandistrogene moxeparvovec-rokl) received expanded FDA approval. This gene therapy is now approved for treating DMD in individuals aged four and older, including non-ambulatory patients. Elevidys works by delivering a gene that produces a functional micro-dystrophin protein to replace the defective dystrophin in DMD patients. The approval was based on clinical trials showing improvements in secondary outcome measures such as motor function and creatine kinase levels.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2032
Product Type Insights:
The report has also provided a comprehensive analysis of the competitive landscape in the global Duchenne muscular dystrophy drugs market. Detailed profiles of all major companies have also been provided.
· FibroGen Inc.
· Italfarmaco S.p.A.
· NS Pharma Inc. (Nippon Shinyaku Co. Ltd.)
· PTC Therapeutics Inc.
· Santhera Pharmaceuticals
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/duchenne-muscular-dystrophy-drugs-market
IMARC Group Offer Other Reports:
Optic Neuropathy Market: The 7 major optic neuropathy market reached a value of US$ 3.0 Billion in 2023 and projected the 7MM to reach US$ 4.4 Billion by 2034, exhibiting a growth rate (CAGR) of 3.46% during the forecast period from 2024 to 2034.
Marburg Virus Disease Market: The 7 major marburg virus disease market is expected to exhibit a CAGR of 5.5% during the forecast period from 2024 to 2034.
Prurigo Nodularis Market: The 7 major prurigo nodularis market is expected to exhibit a CAGR of 3.07% during the forecast period from 2024 to 2034.
Recurrent Glioblastoma Market: The 7 major recurrent glioblastoma market is expected to exhibit a CAGR of 5.85% during the forecast period from 2024 to 2034.
Lymphoproliferative Disorders Market: The 7 major lymphoproliferative disorders market is expected to exhibit a CAGR of 7.32% during the forecast period from 2024 to 2034.
Hyperlipoproteinemia Type ii Market: The 7 major hyperlipoproteinemia type ii market is expected to exhibit a CAGR of 6.79% during the forecast period from 2024 to 2034.
Large Granular Lymphocytic Leukemia Market: The 7 major large granular lymphocytic leukemia market reached a value of US$ 6.5 Billion in 2023 and projected the 7MM to reach US$ 24.5 Billion by 2034, exhibiting a growth rate (CAGR) of 12.81% during the forecast period from 2024 to 2034.
Desmoid Tumors Market: The 7 major desmoid tumors market reached a value of US$ 1.7 Billion in 2023 and projected the 7MM to reach US$ 3.2 Billion by 2034, exhibiting a growth rate (CAGR) of 5.61% during the forecast period from 2024 to 2034.Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The duchenne muscular dystrophy drugs market size reached a value of USD 2.3 Billion in 2023. Looking forward, the market is expected to reach USD 5.5 Billion by 2032, exhibiting a growth rate (CAGR) of 9.96% during 2024-2032.
The market is driven by ongoing advancements and innovations in treatment surgeries, and the development of exon-skipping therapies, such as eteplirsen and golodirsen, which target specific genetic mutations associated with DMD. Additionally, there is a growing emphasis on combination therapies and personalized medicine approaches in the DMD market.
Advancements in Exon-Skipping Therapies: Driving the Duchenne Muscular Dystrophy Drugs Market
Exon-skipping therapies have emerged as a transformative approach in the treatment of Duchenne muscular dystrophy (DMD), aiming to address the underlying genetic cause of the disease. DMD is caused by mutations in the dystrophin gene, resulting in the absence or dysfunction of the dystrophin protein essential for muscle function. Exon-skipping therapies work by modulating RNA splicing to skip specific exons in the dystrophin gene transcript, enabling the production of a shorter but functional dystrophin protein fragment. Moreover, the approval of eteplirsen (Exondys 51) and golodirsen (Vyondys 53) by the U.S. Food and Drug Administration (FDA) marked significant milestones in DMD treatment. Eteplirsen targets exon 51 mutations, while golodirsen targets exon 53 mutations, collectively covering approximately 30% of DMD patients who have amenable mutations. These therapies have shown the ability to increase dystrophin expression in muscle fibers, potentially improving muscle function and slowing disease progression.
Request a PDF Sample Report: https://www.imarcgroup.com/duchenne-muscular-dystrophy-drugs-market/requestsample
Additionally, ongoing research focuses on expanding the applicability of exon-skipping therapies to include additional exons and developing new compounds with improved efficacy and delivery mechanisms. Advances in RNA-targeting technologies, such as antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), continue to enhance the specificity and efficiency of exon-skipping therapies. Moreover, efforts are underway to optimize dosing regimens and address challenges such as immune responses and delivery to target tissues. Apart from this, clinical trials are exploring the long-term safety and efficacy of exon-skipping therapies, aiming to demonstrate sustained benefits in delaying disease progression and preserving muscle function over extended periods. The evolution of these therapies represents a significant shift towards personalized medicine in DMD, tailoring treatments based on individual genetic profiles and mutations. As research progresses, exon-skipping therapies hold promise in transforming the management of DMD by addressing the root cause of the disease and potentially offering disease-modifying effects.
Gene Therapy Innovations: Contributing to Market Expansion
Gene therapy has emerged as a promising frontier in the treatment of Duchenne muscular dystrophy (DMD), offering the potential to provide a one-time, curative treatment by replacing or repairing defective genes responsible for the disease. DMD is characterized by mutations in the dystrophin gene, resulting in the absence or deficiency of dystrophin protein in muscle cells. Gene therapy approaches aim to deliver functional copies of the dystrophin gene directly to affected muscle tissues, restoring dystrophin production and potentially halting disease progression. Moreover, several gene therapy strategies are under investigation, with adeno-associated virus (AAV) vectors being commonly used to deliver therapeutic genes into muscle cells. These vectors are engineered to be safe and effective in transferring genetic material, with ongoing efforts focused on optimizing vector design, enhancing gene expression levels, and minimizing immune responses. Furthermore, recent clinical trials and preclinical studies have shown promising results for gene therapy in DMD. For example, early-stage trials have demonstrated increased dystrophin production and improvements in muscle strength and function in treated patients.
In addition to this, advances in genome editing technologies, such as CRISPR-Cas9, offer additional opportunities to precisely edit the dystrophin gene within muscle cells, potentially correcting specific mutations responsible for DMD. Challenges in gene therapy for DMD include optimizing delivery methods to ensure widespread distribution of therapeutic genes throughout muscles, minimizing immune responses to viral vectors, and achieving long-term durability of treatment effects. Research efforts are focused on addressing these challenges through innovative vector engineering, immune modulation strategies, and advancements in genome editing techniques. Besides this, the evolution of gene therapy in DMD represents a paradigm shift towards curative treatments that could transform the lives of patients and families affected by the disease. Continued research and clinical development are essential to further refine gene therapy approaches, expand treatment options, and ultimately provide effective therapies for all individuals living with DMD.
Emerging Combination Therapies:
Combination therapies are emerging as a promising strategy in the Duchenne muscular dystrophy (DMD) drugs market, aiming to synergistically target multiple disease pathways and enhance therapeutic outcomes. DMD is a complex genetic disorder characterized by progressive muscle degeneration and systemic complications, necessitating a multifaceted treatment approach. Moreover, current research explores combinations of exon-skipping therapies with supportive care interventions, such as corticosteroids, to improve muscle function and quality of life for patients. Corticosteroids, such as prednisone and deflazacort, have been the standard of care for managing DMD by reducing inflammation and delaying disease progression. When combined with exon-skipping therapies, corticosteroids may provide complementary benefits in preserving muscle strength and function.
Another area of interest is the combination of exon-skipping therapies with emerging modalities like gene editing technologies. Gene editing tools, such as CRISPR-Cas9, offer the potential to precisely edit the dystrophin gene within muscle cells, correcting specific mutations and enhancing dystrophin production. Preclinical studies have shown promise in improving muscle function and delaying disease progression in animal models of DMD, highlighting the potential synergies of combining exon-skipping with gene editing therapies. Furthermore, researchers are exploring combinations of pharmacological agents targeting different aspects of DMD pathology, such as inflammation, fibrosis, and mitochondrial dysfunction. These approaches aim to provide comprehensive disease management and improve outcomes for patients with DMD by addressing multiple disease mechanisms simultaneously. Besides this, the development of combination therapies in DMD is driven by a deeper understanding of disease complexity and the need for integrated treatment strategies. Clinical trials are underway to evaluate the safety, efficacy, and synergistic effects of these combinations, optimize treatment regimens, and improve long-term outcomes for individuals living with DMD.
Buy Full Report: https://www.imarcgroup.com/checkout?id=6715&method=502
Leading Companies in the Duchenne Muscular Dystrophy Drugs Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Duchenne muscular dystrophy drugs market, several leading companies are at the forefront for their innovative therapies and significant contributions to the field. Some of the major players include Sarepta Therapeutics Inc., Santhera Pharmaceuticals, and PTC Therapeutics Inc. These companies are actively involved in research and development to introduce new treatments and improve existing therapies, significantly impacting the lives of patients with Duchenne muscular dystrophy.
Sarepta is a significant player in the DMD and is known for its focus on genetic therapies. In June 2024, the FDA expanded the approval of Sarepta Therapeutics' gene therapy, Elevidys (delandistrogene moxeparvovec-rokl), for treating Duchenne muscular dystrophy (DMD) to include children aged 4 and above. This approval now covers both ambulatory and non-ambulatory patients with a confirmed mutation in the DMD gene.
Santhera is involved in the development of vamorolone, a novel steroid alternative, which has been launched in collaboration with Catalyst Pharmaceuticals in the United States. Also, in February 2024, Santhera published full results from its Phase 3 VISION-DMD trial, confirming the efficacy and safety of vamorolone over 48 weeks in boys with DMD. Higher doses of vamorolone (6 mg/kg/day) showed significant improvements in motor outcomes compared to lower doses. The drug also demonstrated a favorable safety profile with fewer adverse events compared to prednisone, including better growth trajectories and weight management.
Apart from this, PTC Therapeutics Inc. focuses on small molecule drugs that target genetic disorders, including DMD. In July 2024, PTC Therapeutics announced plans to submit a new drug application to the FDA for ataluren (Translarna) as a treatment for DMD caused by nonsense mutations. This follows previous conditional approval in Europe and recent discussions with the FDA to address earlier concerns regarding evidence for the drug's efficacy.
Request for customization: https://www.imarcgroup.com/request?type=report&id=6715&flag=E
Regional Analysis:
The major markets for Duchenne muscular dystrophy drugs include North America, Asia Pacific, Europe, Latin America, and the Middle East and Africa. According to projections by IMARC, North America accounted for the largest market share. This can be attributed to the rising investment in research and development to create innovative treatments for DMD, surging emphasis on personalized medicines, and collaborations between pharmaceutical companies.
Moreover, In March 2024, the FDA approved Duvyzat (givinostat), an oral histone deacetylase (HDAC) inhibitor for treating DMD in patients aged six and older. This approval followed a priority review and was based on positive results from a Phase 3 clinical trial, which demonstrated that Duvyzat significantly slowed the decline in muscle function. The most common side effects include diarrhea, abdominal pain, reduced platelet counts, and increased triglycerides.
Besides this, in June 2024, Sarepta Therapeutics' Elevidys (delandistrogene moxeparvovec-rokl) received expanded FDA approval. This gene therapy is now approved for treating DMD in individuals aged four and older, including non-ambulatory patients. Elevidys works by delivering a gene that produces a functional micro-dystrophin protein to replace the defective dystrophin in DMD patients. The approval was based on clinical trials showing improvements in secondary outcome measures such as motor function and creatine kinase levels.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2032
Product Type Insights:
- Corticosteroids
- Prednisolone
- Prednisone
- Deflazacort
- Pain Management Drugs
- Hospitals
- Clinics
- Home Care Settings
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
The report has also provided a comprehensive analysis of the competitive landscape in the global Duchenne muscular dystrophy drugs market. Detailed profiles of all major companies have also been provided.
· FibroGen Inc.
· Italfarmaco S.p.A.
· NS Pharma Inc. (Nippon Shinyaku Co. Ltd.)
· PTC Therapeutics Inc.
· Santhera Pharmaceuticals
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/duchenne-muscular-dystrophy-drugs-market
IMARC Group Offer Other Reports:
Optic Neuropathy Market: The 7 major optic neuropathy market reached a value of US$ 3.0 Billion in 2023 and projected the 7MM to reach US$ 4.4 Billion by 2034, exhibiting a growth rate (CAGR) of 3.46% during the forecast period from 2024 to 2034.
Marburg Virus Disease Market: The 7 major marburg virus disease market is expected to exhibit a CAGR of 5.5% during the forecast period from 2024 to 2034.
Prurigo Nodularis Market: The 7 major prurigo nodularis market is expected to exhibit a CAGR of 3.07% during the forecast period from 2024 to 2034.
Recurrent Glioblastoma Market: The 7 major recurrent glioblastoma market is expected to exhibit a CAGR of 5.85% during the forecast period from 2024 to 2034.
Lymphoproliferative Disorders Market: The 7 major lymphoproliferative disorders market is expected to exhibit a CAGR of 7.32% during the forecast period from 2024 to 2034.
Hyperlipoproteinemia Type ii Market: The 7 major hyperlipoproteinemia type ii market is expected to exhibit a CAGR of 6.79% during the forecast period from 2024 to 2034.
Large Granular Lymphocytic Leukemia Market: The 7 major large granular lymphocytic leukemia market reached a value of US$ 6.5 Billion in 2023 and projected the 7MM to reach US$ 24.5 Billion by 2034, exhibiting a growth rate (CAGR) of 12.81% during the forecast period from 2024 to 2034.
Desmoid Tumors Market: The 7 major desmoid tumors market reached a value of US$ 1.7 Billion in 2023 and projected the 7MM to reach US$ 3.2 Billion by 2034, exhibiting a growth rate (CAGR) of 5.61% during the forecast period from 2024 to 2034.Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800