AnaptysBio, Inc.
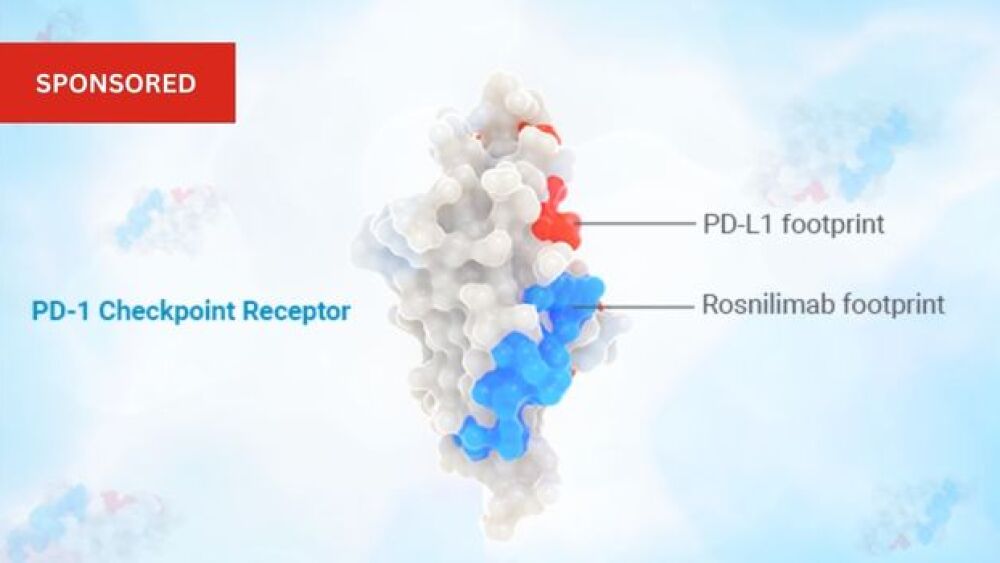
AnaptysBio is a clinical-stage biotechnology company focused on delivering innovative immunology therapeutics. We are developing immune cell modulators, including two checkpoint agonists in clinical-stage development, for autoimmune and inflammatory disease: rosnilimab, our PD-1 agonist program in a Phase 2 trial for the treatment of moderate-to-severe rheumatoid arthritis; and ANB032, our BTLA agonist program, currently in a Phase 2b trial for the treatment of moderate-to-severe atopic dermatitis. Our preclinical immune cell modulator portfolio includes ANB033, an anti-CD122 antagonist antibody for the treatment of autoimmune and inflammatory diseases. In addition, AnaptysBio has developed two cytokine antagonists available for out-licensing: imsidolimab, an anti-IL-36R antagonist, in Phase 3 for the treatment of generalized pustular psoriasis, or GPP, and etokimab, an anti-IL-33 antagonist for the treatment of respiratory disorders that is Phase 2/3 ready. AnaptysBio has also discovered multiple therapeutic antibodies licensed to GSK in a financial collaboration for immune-oncology, including an anti-PD-1 antagonist antibody (Jemperli (dostarlimab-gxly)), an anti-TIM-3 antagonist antibody (cobolimab, GSK4069889) and an anti-LAG-3 antagonist antibody (GSK4074386).
Our corporate vision is to transform patient health by delivering innovative immunology therapeutics. Find out more about us by following us on Twitter.