Food and Drug Administration (FDA)
NEWS
The FDA will still allow continued distribution of sitagliptin containing NTTP with an acceptable intake limit of 37 ng per day and up to 246.7 ng per day.
The FDA has had a busy week, accepting drug applications, approving clinical trials and granting various special designations for Gamida Cell, Cellectis, Scynexis & more.
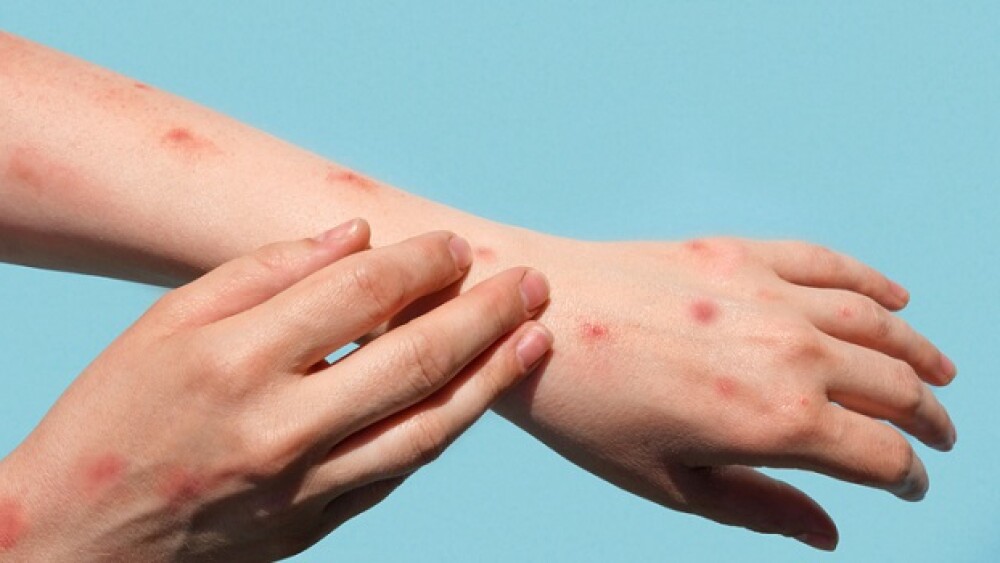
With more than 6,600 cases of monkeypox in the United States, the Biden administration declared the viral outbreak a public health emergency as cases continue to rise across the nation.
Experts from U.S. health regulatory agencies have announced plans to conduct a clinical trial to assess the safety and efficacy of TPOXX (tecovirimat) as an antiviral for monkeypox.
The FDA has placed a clinical hold on Beam Therapeutics’ leukemia/lymphoma therapy and has lifted the hold on Celyad’s CAR-T candidate for colorectal cancer.
IDRx aims to create highly selective and aligned drug combinations to stop key tumor escape mechanisms that will support prolonged and durable responses to therapy.
The decision comes after the FDA put NUV-422, its candidate for high-grade gliomas, on a partial clinical hold, citing safety concerns.
The probe will be led by investigators experienced in determining whether or not entities have defrauded consumers, investors or government agencies.
On Wednesday, Swiss women’s health company ObsEva announced that it is dropping linzagolix, its leading candidate for uterine fibroids.
JOBS
IN THE PRESS