Engage Surgical, a privately held orthopedic implant company, announces FDA 510 clearance of the only cementless partial knee system available in the USA.
ORLANDO, Fla., July 1, 2020 /PRNewswire/ -- Engage Surgical, a privately held orthopedic implant company, announces FDA 510(k) clearance of the only cementless partial knee system available in the USA.
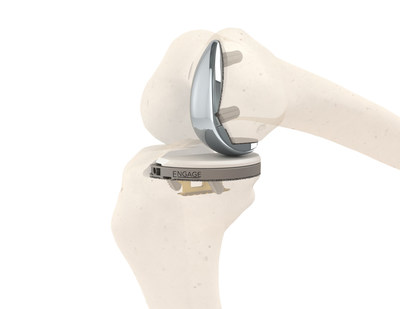
The EngageTM Partial Knee system offers porous implants for promoting biological fixation on both the tibia and femur. The 3-D printed Tibial Tray features the patented Engage™ Anchor technology that provides immediate stability at the tibial interface and the titanium Affinium3D™ ultra-porous bone ingrowth surface that promotes long-term cementless biological fixation.
The system features a simple, reproducible, ligament-guided surgical technique inspired by robotic methods. However, by using precise manual instrumentation, the Engage technique is able to achieve accurate implant placement and desired ligament balance, without requiring the added setup time and extra expense associated with robotic and navigation techniques.
According to Edwin Su, MD, from the Hospital for Special Surgery (HSS) in New York, “The EngageTM Partial Knee system fills a current gap in the treatment of medial compartment arthritis. Currently unicompartmental knee replacements are best suited for my youngest, most active patients. The disruptive technology incorporated into the Engage implant takes it to the next level, with osseointegration of the implant offering the potential for greater implant longevity, while avoiding the pitfalls of cement.”
Andrew Pearle, MD, Chief of Sports Medicine at HSS adds, “Without the need for bone cement, the EngageTM implant system provides instant stability and enhanced compression while also offering long-term biologic fixation. The intuitive design of the instruments make this the best-in-class ligament-guided technique for partial knee replacement. Coupling this technique with a cementless design is a game changer, particularly for my younger, more active patients. As we return to surgery in our COVID-19 world, a knee implant system that is simple to use and minimizes time in the operating room is particularly attractive. Indeed, the efficiency of the EngageTM implant system is ideal for use in the ambulatory surgery center setting. I am excited to use this system in my partial knee arthroplasty patients and returning them to their athletic activities.”
Drs. Su and Pearle with the help of other leading joint arthroplasty and sports medicine surgeons teamed with Engage Surgical to bring to life the 32 issued patents protecting the technology.
Dan Justin, CEO and Co-Founder, explains: “Before founding our company, we asked knee surgeons what problems we could help solve. Repeatedly, surgeons mentioned that active patients are more satisfied with their knee function when healthy articular cartilage and ligament balance is preserved. A stable, non-cemented, ligament preserving, fixed bearing partial knee system was needed. To solve this problem, we brought together an experienced team of surgeons, engineers, and manufacturers who are proud to introduce this groundbreaking system.”
The EngageTM Partial Knee system, FDA cleared for both cementless or cemented fixation, is available first to surgeons at HSS and affiliated ambulatory surgical centers, and to select surgeons at participating facilities throughout the country.
Visit www.EngageSurgical.com to learn more about the technology behind the EngageTM Partial Knee system and Engage Surgical.
About Engage Surgical
Engage Surgical was founded by a team of leading surgeons and engineers with the goal of providing better outcomes for active arthroplasty patients. Engage Surgical prides itself in being more than just an implant technology company, but a multi-disciplinary team focused on clinically relevant problem-solving. The company’s mission is to listen to surgeons and support them with the technical and capital resources needed to develop arthroplasty products that address clinical needs.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/engage-surgical-announces-fda-510k-clearance-and-limited-market-release-of-the-cementless-engage-partial-knee-system-301086541.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/engage-surgical-announces-fda-510k-clearance-and-limited-market-release-of-the-cementless-engage-partial-knee-system-301086541.html
SOURCE Engage Surgical