The FDA has launched a new super office to prepare for myriad decisions on cell and gene therapies, including the potential first CRISPR therapy and the first gene therapy for Duchenne muscular dystrophy.
Pictured: An FDA sign in front of a brick building/iStock, JHVEPhoto
Over the past few years, the pipeline for cell and gene therapies has been increasing at a rapid pace, and the FDA is now ready, thanks to the establishment of a new, specialized super office.
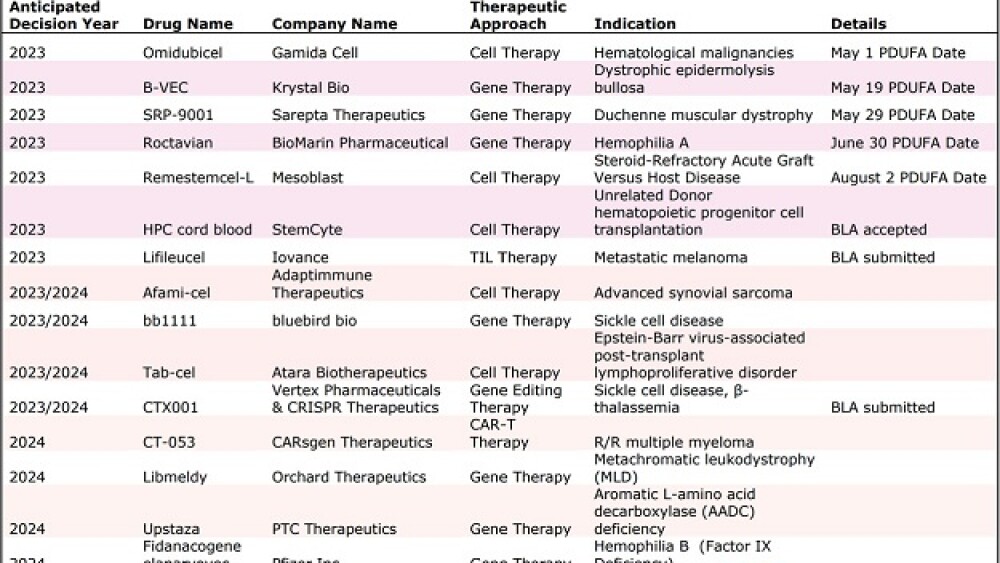
“There could be around 10 total regulatory decisions this year on cell and gene therapies, including up to five decisions on gene therapies for rare genetic diseases alone,” Stephen Majors, global head of communications at the Alliance for Regenerative Medicine (ARM), told BioSpace.
These could include several potential firsts, including the first CRISPR therapy—Vertex Pharmaceuticals and CRISPR Therapeutics’ exa-cel for the treatment of sickle cell disease; and the first gene therapy to treat Duchenne muscular dystrophy (DMD)—Sarepta Therapeutics’ SRP-9001, which could become the second gene therapy to receive approval via the agency’s accelerated approval pathway.
Simon Alfano, an associate partner at McKinsey & Company, said he has noticed a significant increase in the number of cell and gene therapies in clinical development over the past decade.
“There are currently more than 1,000 cell and gene therapies in clinical development in the U.S., with more than 3,000 in pre-clinical development,” Alfano told BioSpace.
“Based on past clinical attrition rates,” he continued, “this level of clinical activity suggests an acceleration of new cell and gene therapy launches towards the second half of this decade.”
Majors said the pressure is now on the FDA to keep up.
Step 1: Launch a Super Office
The Office of Therapeutic Products (OTP) was officially established in March 2023 as the first super office at the FDA’s Center for Biologics Evaluation and Research (CBER), with six sub-offices, 14 divisions and 33 branches. FDA super offices, of which there are three, are designed to streamline workflow processes to improve efficiencies within the agency for the benefit of industry and patients.
“We are currently in the process of filling key positions within OTP that includes a broad spectrum of related scientific fields and medical specialties, including a significant number of hires that will fall into advanced degree categories,” OTP Acting Director Celia Witten told BioSpace. In total, the super office is expected to staff more than 500 people.
CBER Director Peter Marks defended the FDA’s readiness for the looming boom in cell and gene therapy submissions.
“The FDA is prepared to evaluate any IND application associated with a proposed clinical study or use of an investigational cell or gene therapy treatment and determine whether the risks of the proposed study or treatment are reasonable in light of the potential unproven benefits anticipated,” he told BioSpace.
However, the agency, including Marks, is aware that the current regulatory framework for cell and gene therapies needs to be updated to better address the unique challenges these products have introduced.
The agency “is dedicated to developing a regulatory paradigm that can facilitate the development of safe and effective gene therapies, particularly for rare diseases where there is unmet need,” Witten said.
According to experts who spoke with BioSpace, the FDA’s intentions to better support cell and gene therapies come as part of the latest reauthorization of the Prescription Drug User Fee Act (PDUFA). Specifically, the FDA committed as part of PDUFA discussions to enhancing resources for the existing cell and gene therapy program at CBER.
The rapid growth in the development of these products has resulted in a “continuous and sustained increase in the number of applications and meeting requests for a variety of diverse and complex products,” according to a summary of a September 2020 FDA-industry meeting. “This increased workload has forced CBER to limit sponsor interactions, convert many meetings to ‘written responses only,’ and limit external interactions, among other impacts.”
Majors added that the organization has heard from its members about delays “here and there” in interactions with CBER.
Representatives Brett Guthrie (R-KY) and Anna G. Eshoo (D-CA) sent a letter to the agency in March 2023 raising concerns about an increase in clinical holds that the FDA “has recently issued delaying a proposed clinical investigation or suspending an ongoing investigation” for cell and gene therapies.
The FDA also recently missed its PDUFA target date for Sarepta’s gene therapy, requesting more time.
During a recent American Society of Gene & Cell Therapy (ASGCT) meeting, Marks said that CBER was authorized under PDUFA VII to hire up to another 125 reviewers, most of which are “going into cell and gene therapy.”
The hiring commitments and other responsibilities set forth under PDUFA VII are what led the agency to transition CBER’s former Office of Tissues and Advanced Therapies (OTAT) into the new OTP, Kenneth Ndugga-Kabuye, vice president of cell and gene therapy at Premier Research, told BioSpace. “The creation of the office is an attempt to be more responsive, more adaptive, more nimble,” he said.
Step 2: Modernize the Regulatory Paradigm
The establishment of the OTP was just the first step toward keeping up with the growing demand for regulatory review of cell and gene therapies, however.
According to Alfano, the number of early-stage pipeline assets has grown by more than 50% since 2020, while more than 100 late-stage Phase III trials and 600 Phase II trials are currently underway.
Some of these therapies have already made their way to market, with CBER approving a total of 29 cell and gene therapies since the first approval in 2010. And the pace is not likely to slow anytime soon.
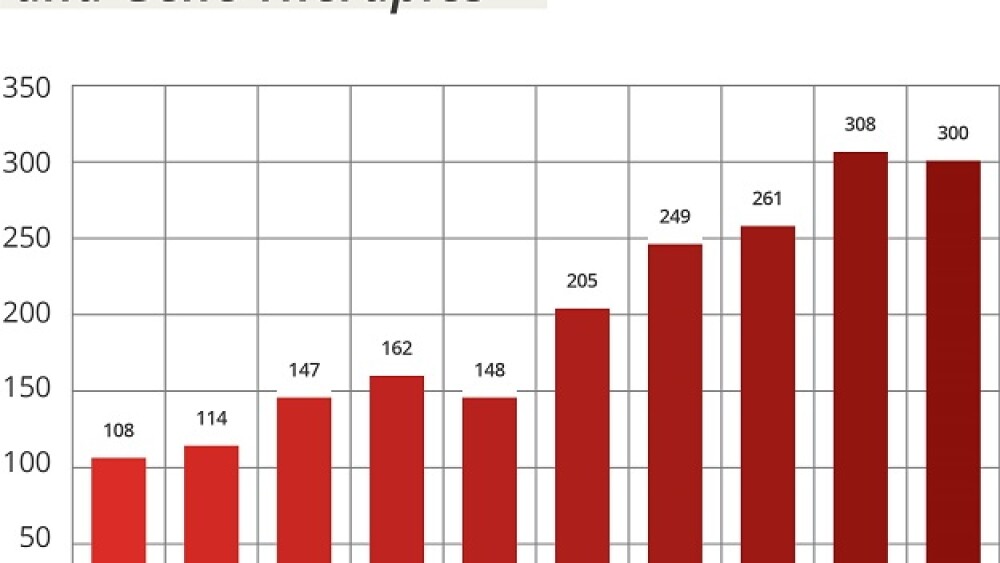
The number of investigational new drug (IND) applications for cell and gene therapies has more than doubled since 2017 (see figure), the FDA stated in its justification for requesting a 10% bump in its budget as part of President Joe Biden’s FY 2024 budget proposal.
Greg Meyer, vice president of regulatory affairs at Premier Consulting, a Premier Research business unit, said this quickening pace can be attributed in part to evolving manufacturing technologies. “It’s stunning what the turnaround time is,” he told BioSpace. “You can do a lot of development work very quickly.”
Thus, while the new super office with its sizeable staff is a good start, it is likely to be insufficient in the long run, experts told BioSpace. The regulatory side and the access and reimbursement side of the cell and gene therapy (CGT) framework need to be modernized to ensure these therapies reach patients, Majors said.
“The creation of OTP and its transition into a super office are signals to us that the FDA is taking those steps to modernize and [that it] views cell and gene therapies as an extremely important part of the future medical landscape.”
Majors and others highlighted additional areas of concern they believe modernization should address: standardization, chemistry, manufacturing and controls (CMC), collaboration and expertise.
“Quickly bringing safe and effective cell and gene therapies to market to cure as many of these diseases as possible will require regulatory frameworks that leverage efficiencies, maximize standardization and make the best use of existing data—at each step of the development process,” Karin Hoelzer, director of policy and regulatory affairs at the National Organization for Rare Disorders, told BioSpace.
Meanwhile, David Barrett, CEO of ASGCT, emphasized the need to adapt the CMC framework, which was originally designed for small molecule drugs.
“The development of CGT products is different—the final CMC data for them often comes later in the products’ lifecycles,” Barrett told BioSpace.
Ndugga-Kabuye echoed Hoelzer and Barrett in stating that the standardization of CMC considerations should be a top priority.
Witten, who also serves as CBER’s deputy director, said the FDA is aware of these concerns and is “steadfastly committed to working with stakeholders . . . to foster beneficial innovation in the field of cell and gene therapies in order to help make the development and approval of these innovative products more efficient.”
However, she added that there are currently no changes to the regulatory review process for submissions to OTP. “Applications continue to be routed and reviewed within the current process.”
Indeed, this transition will take time, experts acknowledge. “Both regulators and developers are kind of learning as we go because we’ve never done this before,” Majors said.
But the changes are necessary, added Barrett. “It’s a truly amazing time for CGT research, and we need to address regulatory challenges in pace with scientific progress. New models will be necessary for patients to get life-saving treatments.”
Ana Mulero is a freelance writer based in Puerto Rico. She can be reached at anacmulero@outlook.com and @anitamulero on Twitter.
Correction (June 12): This story has been updated from its original version to state that Stephen Majors is global head of communications at the Alliance for Regenerative Medicine (ARM), not director of public affairs, and to change the source of data for new INDs from ARM to FDA.