Citing an unnamed FDA insider, the regulator is in the process of devising a plan to enable clinical testing on a larger scale.
University of Maryland School of Medicine/EPA/Shutterstock
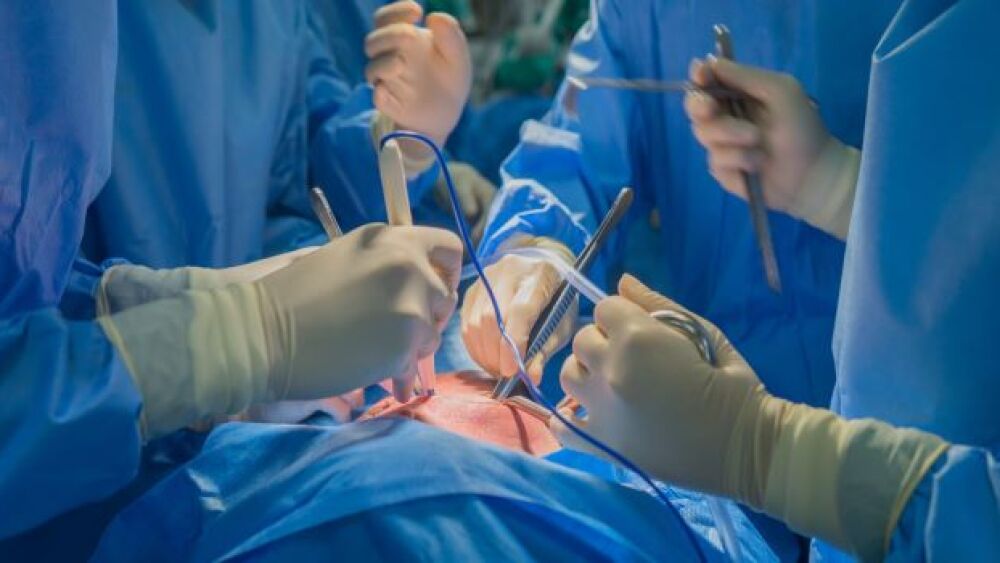
The U.S. Food and Drug Administration has reportedly given the green light to begin clinical trials of pig heart transplantation in humans.
Citing an unnamed FDA insider, the Wall Street Journal reports that the regulator is in the process of devising a plan to enable clinical testing on a larger scale.
In January, Maryland Medical Center doctors successfully transplanted a pig’s heart into a critically ill man to extend his life. The patient died two months after, but the outcome sparked interest in the medical community on the procedure’s strong potential for long-term, sustainable success with further study.
In the paper titled “First pig-to-human heart transplant: what can scientists learn?” published in Nature, scientists for the first time performed xenotransplantation on 57-year-old David Bennett, who had been on cardiac support for two months prior to the surgery.
Bennett was not eligible for a mechanical heart pump because he had been experiencing an irregular heartbeat. He was also not eligible for a human transplant due to his history of non-compliance with doctors’ orders. The FDA allowed xenotransplantation via a “compassionate use” authorization.
The University of Maryland School of Medicine (UMSOM) scientists then used a heart from a genetically modified pig that biotech company Revivicor created. To make the pig heart ready for the procedure, the company removed three pig genes that might trigger attacks from the human immune system. They also added six human genes that may help the body be more receptive to the organ.
Bennett survived for two months, even managing to spend time with family and watch the Super Bowl, before eventually dying on March 8.
“We are devastated by the loss of Mr. Bennett. He proved to be a brave and noble patient who fought all the way to the end,” Bartley P. Griffith, MD, who surgically transplanted the pig heart into the patient at UMMC, said.
“As with any first-in-the-world transplant surgery, this one led to valuable insights that will hopefully inform transplant surgeons to improve outcomes and potentially provide lifesaving benefits to future patients,” Griffith added.
As of this writing, the FDA has yet to issue a statement about its future plans for pig-to-human heart experimental transplants. It also remains unknown when the regulator intends to begin planning, as proposals from researchers would be assessed on a case-to-case basis. If it does happen, the trial could be a revolutionary step toward addressing the massive global lack of human donor organs. It also offers hope to patients who may not be qualified for human organ transplantation.