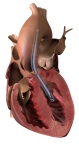
Three cardiac surgeons at Cleveland Clinic, Hackensack Meridian Health and Cedars-Sinai Medical Center are the first in the United States to implant Abiomed’s newest heart pump, the Impella 5.5 with SmartAssist. Ed Soltesz, MD, Mark Anderson, MD, and Danny Ramzy, MD, have each successfully implanted multiple pumps during cardiac procedures at their hospitals..

DANVERS, Mass.--(BUSINESS WIRE)-- Three cardiac surgeons at Cleveland Clinic, Hackensack Meridian Health and Cedars-Sinai Medical Center are the first in the United States to implant Abiomed’s (NASDAQ: ABMD) newest heart pump, the Impella 5.5 with SmartAssist. Ed Soltesz, MD, Mark Anderson, MD, and Danny Ramzy, MD, have each successfully implanted multiple pumps during cardiac procedures at their hospitals.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191031005267/en/
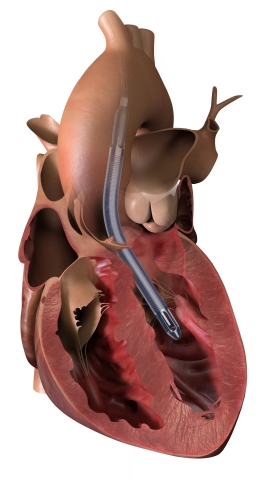
The Impella 5.5 with SmartAssist pulls blood from the left ventricle through an inlet area near the tip of the pump and expels blood from the catheter into the ascending aorta. (Photo: Business Wire)
Mark Anderson, MD, chief of the Division of Cardiac Surgery and cardiothoracic surgeon at the Heart and Vascular Hospital at Hackensack University Medical Center and Hackensack Meridian Health said, “The Impella 5.5 is a promising new option for heart failure patients in cardiogenic shock. It is easy to implant through the axillary artery to avoid opening the chest or, if the chest is already open during a surgical procedure, it can be implanted directly into the anterior aorta. Additionally, with SmartAssist, I can monitor the left ventricle function of my patient in real time in the ICU.”
The Impella 5.5 with SmartAssist has received the U.S. Food and Drug Administration’s (FDA) highest level of approval for safety and efficacy in the therapy of cardiogenic shock for up to 14 days. It is a temporary heart pump that is:
- Minimally invasive, eliminating the need for a sternotomy or coring of the left ventricle
- Designed for heart surgeons, implanted via the axillary artery or the anterior aorta
- Forward flow, to provide the patient with coronary flow and end organ perfusion
- Fully unloading, to reduce the heart’s oxygen demand and work
- Equipped with SmartAssist, designed to provide weaning algorithms to optimize survival and native heart recovery
Impella 5.5 with SmartAssist delivers peak flows of greater than 6 liters per minute. A motor housing that is thinner and 45% shorter than the Impella 5.0 improves ease of pump insertion through the vasculature.
“The Impella 5.5 with SmartAssist is an innovative pump designed for heart surgeons to treat heart failure patients in cardiogenic shock and optimize heart recovery with SmartAssist real-time feedback in the ICU and on surgeons’ smart phones with Impella Connect,” said Michael R. Minogue, Chairman, President and Chief Executive Officer of Abiomed.
The Impella 5.5 with SmartAssist is being introduced in the United States through a controlled rollout at hospitals with established heart recovery protocols. It received CE marking approval in Europe in April 2018 and was introduced in Germany through a similar controlled rollout.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA PMA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5™ with Smart Assist® are U.S. FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP® is U.S. FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. Impella is the most studied mechanical circulatory support device in the history of the FDA with real world clinical data on more than 100,000 patients and more than 550 peer-reviewed publications.
In Europe, the Impella 2.5, Impella CP and Impella CP with SmartAssist are CE marked for treatment of high-risk PCI and AMI cardiogenic shock patients for up to 5 days. Impella 5.0 and Impella LD are CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 10 days. The Impella 5.5™ heart pump is CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 30 days. The Impella RP is CE marked to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia.
To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit www.impella.com.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit www.abiomed.com.
Abiomed, Impella, Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, and Impella Connect are registered trademarks of Abiomed, Inc., and are registered in the U.S. and certain foreign countries. Impella BTR, Impella 5.5, Impella ECP, CVAD Study, and SmartAssist are pending trademarks of Abiomed, Inc.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of Abiomed's existing and new products, the company's progress toward commercial growth, and future opportunities and expected regulatory approvals. The company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including uncertainties associated with development, testing and related regulatory approvals, including the potential for future losses, complex manufacturing, high quality requirements, dependence on limited sources of supply, competition, technological change, government regulation, litigation matters, future capital needs and uncertainty of additional financing, and other risks and challenges detailed in the company's filings with the Securities and Exchange Commission, including the most recently filed Annual Report on Form 10-K and Quarterly Report on Form 10-Q. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. The company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.
View source version on businesswire.com: https://www.businesswire.com/news/home/20191031005267/en/
Contacts
Tom Langford
Director, Communications and Public Relations
978-882-8408
tlangford@abiomed.com
Ingrid Goldberg Ward
Director, Investor Relations
978-646-1590
igoldberg@abiomed.com
Source: Abiomed, Inc.
Smart Multimedia Gallery
The Impella 5.5 with SmartAssist pulls blood from the left ventricle through an inlet area near the tip of the pump and expels blood from the catheter into the ascending aorta. (Photo: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20191031005267/en