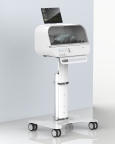
Fitbit announced it has developed a high-quality, low-cost, easy-to-use emergency ventilator, Fitbit Flow, which has obtained Emergency Use Authorization from the U.S. Food & Drug Administration for use during the COVID-19 public health emergency.

Fitbit Flow has been granted Emergency Use Authorization by the FDA
SAN FRANCISCO--(BUSINESS WIRE)-- Fitbit (NYSE: FIT) today announced it has developed a high-quality, low-cost, easy-to-use emergency ventilator, Fitbit Flow, which has obtained Emergency Use Authorization (EUA) from the U.S. Food & Drug Administration (FDA) for use during the COVID-19 public health emergency.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200603005785/en/
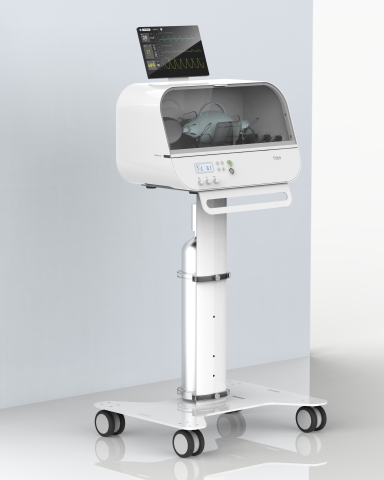
Fitbit Flow, a low-cost, easy-to-use emergency ventilator, builds on standard resuscitator bags, like those used by paramedics, with sophisticated instruments, sensors, and alarms that work together to support automated compressions and patient monitoring. Fitbit Flow has obtained Emergency Use Authorization from the FDA for use during the COVID-19 pandemic. (Photo: Business Wire)
After seeing the global need for ventilators, Fitbit applied its deep in-house expertise in advanced sensor development and hardware design to quickly create Fitbit Flow, an automatic resuscitator inspired by the MIT E-Vent Design Toolbox and based on specifications for Rapidly Manufactured Ventilation Systems. During development and testing, Fitbit consulted with Oregon Health & Science University emergency medicine clinicians caring for COVID-19 patients at OHSU Hospital and worked with the Mass General Brigham Center for COVID Innovation working group on the design to meet the needs of practitioners.
“COVID-19 has challenged all of us to push the boundaries of innovation and creativity, and use everything at our disposal to more rapidly develop products that support patients and the health care systems caring for them,” said James Park, co-founder and CEO of Fitbit. “We saw an opportunity to rally our expertise in advanced sensor development, manufacturing, and our global supply chain to address the critical and ongoing need for ventilators and help make a difference in the global fight against this virus.”
Fitbit Flow builds on standard resuscitator bags, like those used by paramedics, with sophisticated instruments, sensors, and alarms that work together to support automated compressions and patient monitoring. The device is designed to be intuitive and simple to use, potentially helping to reduce the strain on specialized staff who are typically needed to operate a commercial ventilator. Other similar emergency ventilators vary in the combination of features they offer, but Fitbit believes that none delivers all of the attributes of its device at the same lower price range.
According to the New England Journal of Medicine, “U.S. hospitals are already reporting shortages of key equipment needed to care for critically ill patients, including ventilators and personal protective equipment (PPE) for medical staff. Current estimates of the number of ventilators in the United States range from 60,000 to 160,000. No matter which estimate we use, there are not enough ventilators for patients with COVID-19 in the upcoming months.”
“Fitbit Flow is a great example of the incredible innovation that emerges when academia and industry employ problem-based innovation to respond quickly to an important need. COVID-19 is a new illness and we still have much to learn about the progression, treatment, and potential recurrence of this disease. It’s critical that we develop solutions that can help ensure our health systems have the equipment they need now, and in the future if we do see a resurgence of COVID-19,” said David Sheridan,MD, MCR, Assistant Professor of Pediatric Emergency Medicine and Co-Director of Emergency Clinical Innovation Oregon Health & Science University.
Fitbit aims to leverage the company’s vast infrastructure and manufacturing capabilities that currently produces millions of Fitbit devices per year to produce large volumes of these emergency devices quickly. The goal is to supply these devices to health care systems around the world that do not have a sufficient number of traditional commercial ventilators. Fitbit Flow is designed to be used only when a traditional commercial ventilator is not available.1
Fitbit is in talks with state and federal agencies to understand current domestic needs for emergency ventilators and plans to work with U.S. and global aid organizations as well, both today and ahead of any future waves of the virus.
About Fitbit, Inc. (NYSE: FIT)
Fitbit helps people lead healthier, more active lives by empowering them with data, inspiration and guidance to reach their goals. Fitbit designs products and experiences that track and provide motivation for everyday health and fitness. Fitbit’s diverse line of innovative and popular products include Fitbit Charge 4™, Fitbit Charge 3™, Fitbit Inspire HR™, Fitbit Inspire™ and Fitbit Ace 2™ activity trackers, as well as the Fitbit Ionic™ and Fitbit Versa™ family of smartwatches, Fitbit Flyer™ wireless headphones, and Fitbit Aria family of smart scales. Fitbit products are carried in approximately 39,000 retail stores and in 100+ countries around the globe. Powered by one of the world’s largest databases of activity, exercise and sleep data and Fitbit’s leading health and fitness social network, the Fitbit platform delivers personalized experiences, insights and guidance through leading software and interactive tools, including the Fitbit and Fitbit Coach apps, and Fitbit OS for smartwatches. Fitbit’s paid subscription service, Fitbit Premium, uses your unique data to deliver actionable guidance and coaching in the Fitbit app to help you reach your health and fitness goals. Fitbit Health Solutions develops health and wellness solutions designed to help increase engagement, improve health outcomes, and drive a positive return for employers, health plans and health systems.
Fitbit and the Fitbit logo are trademarks or registered trademarks of Fitbit, Inc. in the U.S. and other countries. Additional Fitbit trademarks can be found at www.fitbit.com/legal/trademark-list. Third-party trademarks are the property of their respective owners.
Connect with us on Facebook, Instagram or Twitter and share your Fitbit experience.
Forward Looking Statement
This press release contains forward-looking statements, within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, that involve risks and uncertainties including, among other things, statements about the attributes and pricing of Fitbit Flow. These forward-looking statements are only predictions and may differ materially from actual results due to a variety of factors, including the effects of the highly competitive market in which we operate, including competition from much larger technology companies; any inability to successfully develop and introduce new products, features, and services or enhance existing products and services; product liability issues, security breaches or other defects; the impact of the recent outbreak of the COVID-19 virus; and other factors discussed under the heading “Risk Factors” in our most recent report on Form 10-Q filed with the Securities and Exchange Commission. All forward-looking statements contained herein are based on information available to us as of the date hereof and we do not assume any obligation to update these statements as a result of new information or future events.
____________________________
1 Fitbit Flow has been authorized by FDA under an EUA for use during the COVID-19 public health emergency. It is not FDA cleared or approved.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200603005785/en/
Fitbit, Inc.
Media: Jen Ralls 415-941-0037, PR@Fitbit.com
Source: Fitbit, Inc.
Fitbit Flow, a low-cost, easy-to-use emergency ventilator, builds on standard resuscitator bags, like those used by paramedics, with sophisticated instruments, sensors, and alarms that work together to support automated compressions and patient monitoring. Fitbit Flow has obtained Emergency Use Authorization from the FDA for use during the COVID-19 pandemic. (Photo: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20200603005785/en