Focal Medical, Inc. today announced U.S. Food and Drug Administration (FDA) clearance of an Investigational New Drug (IND) application to initiate a Phase 1b clinical trial of ACT-IOP-003, the Company’s first targeted therapeutic product.
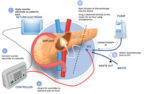
ACT-IOP-003 is an implantable iontophoretic product designed to safely achieve high concentration of gemcitabine in the pancreas, via non-circulatory pathways and with little to no systemic drug exposure or associated toxicity.
Demonstrated dramatic results in an orthotopic patient-derived xenograft murine model of human pancreatic cancer in which 100% of treated tumors averaged 40% volume reduction.
Study expected to start mid-2024.
CARY, N.C.--(BUSINESS WIRE)-- Focal Medical, Inc., (“Focal”) a privately held biopharmaceutical company developing a targeted therapeutic system to treat inoperable tumors and to deliver genomic medicines, today announced U.S. Food and Drug Administration (FDA) clearance of an Investigational New Drug (IND) application to initiate a Phase 1b clinical trial of ACT-IOP-003, the Company’s first targeted therapeutic product. The trial will evaluate the safety and tolerability of targeted delivery of gemcitabine to locally advanced nonresectable (LANR) pancreatic tumors. The clinical trial is expected to start mid-2024.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240109845617/en/
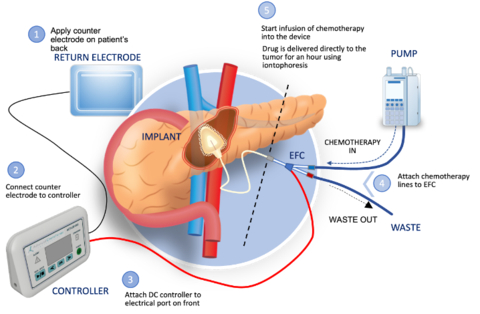
Focal Medical’s Implantable Iontophoresis Chemotherapy Delivery Device with Gemcitabine (ACT-IOP-003) (Photo: Business Wire)
“Pancreatic cancer is a devastating disease with a very poor prognosis,” said Dr. William Daunch, Chief Technology Officer of Focal. “Once a locally advanced tumor is not resectable, treatment options are limited and these patients experience a significantly reduced survival outlook compared to resectable cases. Our thesis, which we have demonstrated in animal models of pancreatic cancer, is that localized delivery of high concentrations of gemcitabine via our implantable iontophoretic device can reduce tumor volume to a point where surgical removal may be possible, while also minimizing systemic exposure and associated toxicity. If successful, we may offer the opportunity of extended survival for the significant number of pancreatic cancer patients presenting with nonresectable disease.”
Pancreatic adenocarcinoma, which represents more than 90 percent of pancreatic cancer diagnoses, is an especially challenging disease to treat. According to The American Cancer Society, the incidence of pancreatic cancer in the U.S. is more than 62,000 cases annually and it represents the third leading cause of cancer death. Patients whose tumors are resectable have the best chance for cure and, for these patients, surgical resection with or without neoadjuvant chemotherapy, is the standard of care. Treatment options for locally advanced nonresectable tumors comprise a variety of systemic chemotherapy regimens, which are rarely curative unless there is dramatic response to chemotherapy that allows the tumor to be resected.
The multi-center, open label, modified dose escalation phase 1b clinical trial will assess the safety, tolerability, and clinical activity of the implantable ACT-IOP-003 targeted therapeutic product delivering gemcitabine directly into the pancreas to treat LANR pancreatic cancer. Eligible patients would be enrolled into one of two cohorts, receiving treatment either once or twice weekly over 8 weeks (approximately 5 patients per cohort). Up to 12 patients may be enrolled in the study which is expected to start mid-2024.
“Clinicians who treat pancreatic cancer need new and more powerful tools in their armamentarium to address this terrible disease,” commented Jen Jen Yeh, M.D., Focal Medical Co-founder and Non-executive Director, and Professor and Vice Chair of Research, Department of Surgery and Director of the Pancreatic Cancer Center of Excellence at the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill. “The novel approach that the Focal Medical system employs to drive gemcitabine directly and selectively into the pancreas may offer new hope for patients.”
Michael Aldridge, CEO of Focal added, “The FDA’s clearance of Focal’s IND for ACT-IOP-003 for pancreatic cancer is an important achievement for the Company. We remain focused on our important mission to provide hope for patients suffering with this devastating disease.”
About Focal Medical
Focal Medical, Inc. is a privately held, biopharmaceutical company developing novel therapeutic products based on its innovative and patent protected targeted therapeutic system. The Company’s lead product is a targeted therapeutic product delivering gemcitabine (an FDA approved chemotherapeutic) actively and directly to the pancreas by non-circulatory pathways to treat pancreatic cancer. Focal Medical is expanding its product focus into therapies for other solid tumors and genomic medicine products. Its products utilize its innovative energy-based targeted therapeutic system. Focal Medical’s patented iontophoresis delivery system enables the internal, site-specific delivery of therapeutics actively, directly, and selectively to the target organ using non-circulatory pathways. The technology thus addresses certain significant challenges and limitations of traditional systemic drug delivery including systemic toxicity and barriers to therapeutic effect. Please visit our website at: www.focalmedical.co.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240109845617/en/
Contacts
Company Contacts:
Michael Aldridge, CEO
(919) 651-4656
maldridge@focalmedical.co
Tony Voiers, COO
(919) 917-7324
tvoiers@focalmedical.co
Investor Contact:
Burns McClellan
Lee Roth / Eric Ando
lroth@burnsmc.com / eando@burnsmc.com
Media Contact:
Burns McClellan
Selina Husain / Robert Flamm, Ph.D.
shusain@burnsmc.com / rflamm@burnsmc.com
Source: Focal Medical, Inc.