GenSight Biologics announces that efficacy and safety data from patients with Leber Hereditary Optic Neuropathy carrying the ND4 mutation treated with lenadogene nolparvovec through early access programs, were presented at the 49th Annual Meeting of the North American Neuro-Ophthalmology Society.
- Efficacy and Safety profile of LUMEVOQ® confirmed in a real-world setting
- Improvement of visual acuity of +22.5 ETDRS letters vs nadir at one year
- Data presented at the NANOS 2023 Annual Meeting, March 14
PARIS--(BUSINESS WIRE)-- Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230314006021/en/
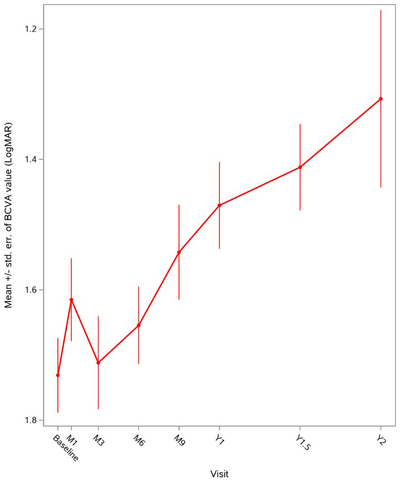
Figure 1: Global evolution of mean BCVA over two years in patients who received LUMEVOQ® in early access programs. (Graphic: Business Wire)
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announces that efficacy and safety data from patients with Leber Hereditary Optic Neuropathy carrying the ND4 mutation (ND4-LHON) treated with lenadogene nolparvovec (LUMEVOQ®, GS010) through early access programs (EAP), were presented at the 49th Annual Meeting of the North American Neuro-Ophthalmology Society (NANOS). The data were collected from EAPs across the US, France, Italy, and the UK.
“It is important to observe that the efficacy and safety data from these early access programs are consistent with what LUMEVOQ showed in its clinical trials and that they confirm LUMEVOQ as being a real option for LHON patients with the ND4 mutation,” commented Catherine Vignal-Clermont, MD, and Head of the Ophthalmology Department at Foundation Hospital Adolphe de Rothschild, Paris, France, and Head of the Ophthalmology, Neuro-Ophthalmology and Oculomotricity Department at the Quinze-Vingts National Eye Hospital, Paris, France. “And I was delighted to share these data with the wider scientific and medical community at the prestigious NANOS Conference.”
Lenadogene nolparvovec was made available through EAPs in the respective countries based on unsolicited requests from clinicians and patients and authorized for use by local regulations. Between August 2018 and March 2022, 63 ND4-LHON patients received intravitreal injections of lenadogene nolparvovec, with 67 percent of patients receiving injections in both eyes (bilateral treatment), while 33 percent of patients received an injection in one eye (unilateral treatment). Individual data from 45 out of the 63 patients who had passed the one-year post-treatment point and performed their one-year visit were pooled and analyzed1.
The data confirmed the efficacy and safety profile of LUMEVOQ® in a real-world setting. The mean change in best-corrected visual acuity (BCVA) at one-year post-treatment in all eyes was an increase of 22.5 ETDRS letters (-0.45 LogMAR) compared to nadir (i.e., the worst visual acuity achieved from baseline to one-year time point). The improvement was better in patients who received bilateral injections with a mean BCVA improvement of 24.5 ETDRS letters (-0.49 LogMAR) versus nadir in comparison to 19.5 ETDRS letters (-0.39 LogMAR) for unilaterally treated patients.
Figure 1: Global evolution of mean BCVA over two years in patients who received LUMEVOQ® in early access programs.
[Graph included above]
| Baseline | Month 1 | Month 3 | Month 6 | Month 9 | Year 1 | Year 1.5 | Year 2 | |
| Number of eyes | 124 | 107 | 107 | 103 | 88 | 90 | 34 | 14 |
Responder analyses demonstrate clinically meaningful improvement in BCVA in a large proportion of patients’ eyes. One year after treatment1, 64 percent of eyes showed an improvement of at least 15 ETDRS letters (‑0.3 LogMAR) from nadir and 60 percent of eyes achieved clinically relevant recovery (CRR) from nadir.
The safety results obtained in the EAPs were consistent with those observed in the clinical studies, showing a favorable safety profile of lenadogene nolparvovec. Notably, intraocular inflammation events reported in LUMEVOQ®-treated eyes were comparable in frequency, intensity, and location to those observed in the clinical studies.
Bernard Gilly, Chief Executive Officer and Co-Founder of GenSight Biologics, said: “These real-world data add to our growing body of clinical evidence in which LUMEVOQ has repeatedly demonstrated its ability to safely improve visual acuity in ND4-LHON patients who are losing their sight. We are now concentrating all our efforts to bring this innovative treatment to the wider ND4-LHON population as soon as possible.”
The efficacy and safety data from EAP for ND4-LHON patients were presented through a poster by Catherine Vignal-Clermont at NANOS Conference being held in Orlando, Florida from March 11-16, 2023.
- Presentation: “Use of Lenadogene Nolparvovec Gene Therapy for Leber Hereditary Optic Neuropathy in Early Access Programs”
- Poster Presentation by Catherine Vignal-Clermont, MD
- Session: Analytical Studies (poster number: 279)
- Tuesday, March 14th, 2023, 6:30 - 7:30 pm EST
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, LUMEVOQ® (GS010; lenadogene nolparvovec), is an investigational compound and has not been registered in any country at this stage; a marketing authorization application is currently under review by the EMA for the treatment of Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease affecting primarily teens and young adults that leads to irreversible blindness. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About LUMEVOQ® (GS010; lenadogene nolparvovec)
LUMEVOQ® (GS010; lenadogene nolparvovec) targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function. “LUMEVOQ” was accepted as the invented name for GS010 (lenadogene nolparvovec) by the European Medicines Agency (EMA) in October 2018. LUMEVOQ® (GS010; lenadogene nolparvovec), is an investigational compound and has not been registered in any country at this stage; a marketing authorization application is currently under review by the EMA.
______________________
1 One year after treatment analysis might be updated in the future with additional evaluable eyes as more patients reach the one-year post-treatment point and perform their one-year visit.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230314006021/en/
GenSight Biologics
Corporate Communications Director
Clothilde Caillet
ccaillet@gensight-biologics.com
LifeSci Advisors
Investor Relations
Guillaume van Renterghem
gvanrenterghem@lifesciadvisors.com
+41 (0)76 735 01 31
RooneyPartners
Media Relations
Jeanene Timberlake
jtimberlake@rooneypartners.com
+1 646-770-8858
Orpheon Finance
Retail Investors
James Palmer
j.palmer@orpheonfinance.com
+33 (0)7 60 92 77 74
Source: GenSight Biologics S.A.
Figure 1: Global evolution of mean BCVA over two years in patients who received LUMEVOQ® in early access programs. (Graphic: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20230314006021/en







