Imricor achieves commercial milestone with first customer contract
Imricor achieves commercial milestone with first customer contract
MINNEAPOLIS--(BUSINESS WIRE)-- Imricor announced today that it signed its first commercialization contract for the Advantage-MR EP Recorder/Stimulator System and supporting Vision-MR devices1 with the Heart Center Dresden in Germany. This agreement places the Heart Center Dresden at the forefront of clinical advancement for MRI-guided cardiac ablations and establishes the hospital as a world leader in the growing field of interventional cardiac magnetic resonance (iCMR).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20181010005779/en/
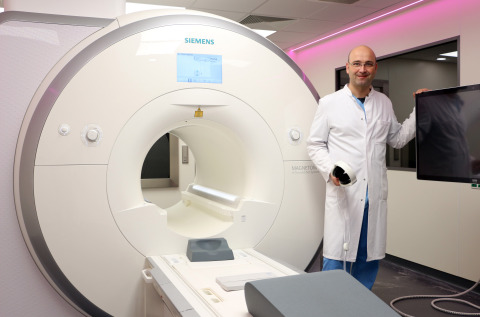
Dr. Christopher Piorkowski in the newly installed iCMR lab at the Heart Center Dresden. (Photo: Heart Center Dresden)
“Here at the Heart Center Dresden, we are proud to have the opportunity and the commitment to pioneer forefront research in the field of cardiac arrhythmia treatment,” said Dr. Christopher Piorkowski, Head of Electrophysiology at the Heart Center Dresden. “Having recently completed the successful installation of one of the most advanced cardiac MRI setups in the world, we are now preparing for the first routine clinical applications of catheter ablation within the MRI. We expect groundbreaking new insights and an innovative push into our understanding and treatment of patients with structural heart disease and related cardiac arrhythmias.”
Imricor’s Advantage-MR System and Vision-MR devices are state-of-the-art cardiac ablation tools that allow physicians to perform procedures under real-time MRI guidance. Unlike conventional x-ray guided procedures, ablations guided by MRI allow physicians to individualize the treatment strategy for each patient’s unique cardiac structure and substrate as well as assess lesion quality and fill gaps in ablation lines during the initial procedure. These benefits have the potential to improve patient outcomes and provide safer, more cost-effective treatment – all in an environment that is free of radiation for both the patient and physician.
Prof. Dr. Bärbel Held, Managing Director of the Heart Center Dresden, commented, “I am very proud, together with Imricor and Dr. Piorkowski, for the heart center in Dresden to introduce and apply such an innovation. For our patients, this will be a big advantage in the treatment of cardiac arrhythmias. This is what the Heart Center Dresden stands for.”
“This is an exciting milestone for both Imricor and the Heart Center Dresden. It marks the beginning of the commercial clinical application of iCMR-guided ablations and a new age for interventional cardiology,” said Steve Wedan, CEO of Imricor. “Prof. Held has a brilliant vision for the future of medicine at Heart Center Dresden, and we couldn’t be more thrilled to be working with Dr. Piorkowski and his extraordinary team as we usher in this new age together.”
1Delivery of Vision-MR devices will commence upon CE mark approval of devices.
About Imricor
Imricor Medical Systems is a privately held company committed to unlocking the potential of interventional cardiac magnetic resonance (iCMR) by delivering MR-compatible systems and devices that allow physicians to perform cardiac ablations under real-time MRI-guidance.
About the Heart Center Dresden
The Heart Center Dresden is a University Hospital owned by the Sana Kliniken AG. The Heart Center is structured into three departments – Cardiac Surgery, Cardiology, and Electrophysiology. Over 650 employees provide in-patient and out-patient services for nearly 25,000 patients per year. In alliance with the Technical University Dresden, medical education as well as research and sciences are integral parts of the portfolio.
CAUTION: The Advantage-MR™ EP Recorder/Stimulator System has received CE mark approval; it has not yet been approved for use in the United States. The Vision-MR™ Ablation Catheter has been approved as an investigational device for clinical studies in Europe. All other Imricor devices are not yet approved for use in humans.
View source version on businesswire.com: https://www.businesswire.com/news/home/20181010005779/en/
Contacts
Imricor
Jodi Lamberti, +1 952-818-8417
info@imricor.com
www.imricor.com
or
Heart Center Dresden
Robert Reuther, +49 351 450 1555
robert.reuther@herzzentrum-dresden.com
www.herzzentrum-dresden.com
Source: Imricor
Smart Multimedia Gallery
Dr. Christopher Piorkowski in the newly installed iCMR lab at the Heart Center Dresden. (Photo: Heart Center Dresden)