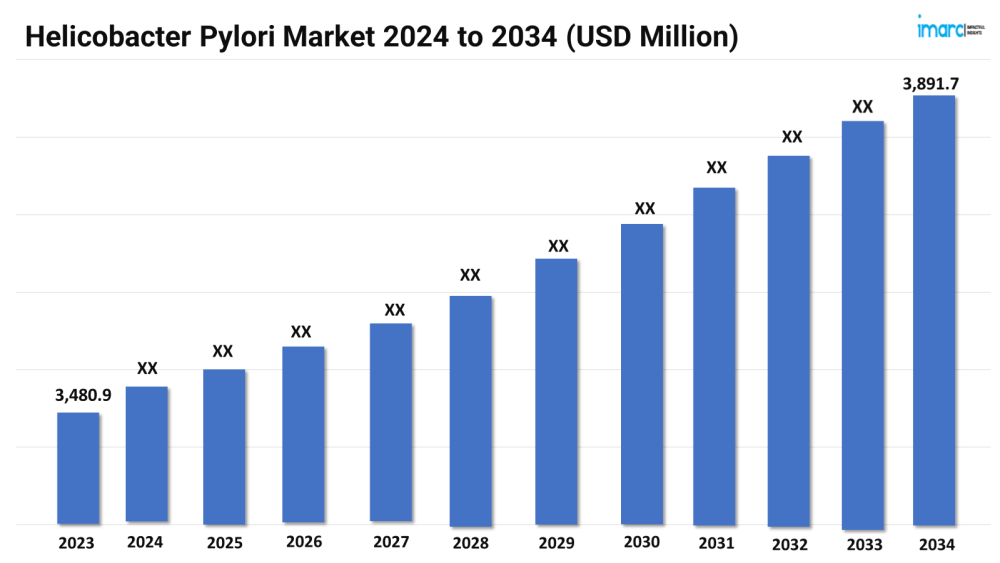
The helicobacter pylori market size reached a value of USD 3,480.9 Million in 2023. Looking forward, the market is expected to reach USD 3,891.7 Million by 2034, exhibiting a growth rate (CAGR) of 1.02% during 2024-2034.
The market is driven by a shift towards non-invasive diagnostic methods, such as serological tests and stool antigen assays, reducing patient discomfort. Additionally, there is a growing focus on combination therapies involving proton pump inhibitors and antibiotics to improve treatment efficacy and reduce antibiotic resistance.
Rise of Non-Invasive Diagnostics: Driving the Helicobacter Pylori Market
The Helicobacter pylori (H. pylori) market has witnessed a notable rise in non-invasive diagnostic methods, marking a pivotal shift in how healthcare professionals diagnose and manage this prevalent bacterial infection. Numerous advancements in diagnostic technologies have introduced several non-invasive alternatives that are increasingly favored for their convenience, accuracy, and patient-friendly nature. One of the primary non-invasive diagnostic tools gaining prominence is the urea breath test (UBT). This test detects the presence of H. pylori by measuring the levels of carbon dioxide in the patient's breath after ingesting a urea solution. UBT is highly sensitive and specific, offering reliable results within a short timeframe, typically within 30 minutes to an hour. It is widely used in clinical settings and is considered a preferred method for diagnosing H. pylori due to its accuracy and non-invasive nature.
Request a PDF Sample Report: https://www.imarcgroup.com/helicobacter-pylori-market/requestsample
Additionally, serological tests have become another valuable non-invasive option. These tests detect antibodies against H. pylori in the patient's blood serum, indicating current or past infection. While serological tests are convenient and accessible, they are primarily used for screening purposes rather than for confirming active infection due to their inability to distinguish between past and current infections reliably. The adoption of non-invasive diagnostics in the H. pylori market is driven by several factors. Patients prefer these methods due to their minimal discomfort and avoidance of invasive procedures. Healthcare providers benefit from their efficiency and ease of use, enabling quicker diagnosis and initiation of appropriate treatment. Moreover, non-invasive tests are particularly valuable in primary care settings and resource-limited regions where access to specialized equipment and procedures may be limited. Moreover, the rise of non-invasive diagnostics represents a significant advancement in the management of H. pylori infections, improving patient care and streamlining healthcare practices. As technology continues to evolve, these methods are expected to play an increasingly critical role in early detection and effective treatment strategies for H. pylori, ultimately enhancing patient outcomes and reducing the overall burden of gastrointestinal diseases globally.
Combination Therapies: Contributing to Market Expansion
Combination therapies have revolutionized the treatment landscape for Helicobacter pylori infections, offering multifaceted approaches to overcome antibiotic resistance and enhance eradication rates. Traditionally, standard therapies involved triple combinations of proton pump inhibitors (PPIs) with antibiotics such as clarithromycin, amoxicillin, or metronidazole. However, the efficacy of these regimens has been increasingly challenged by the rise of antibiotic-resistant strains of H. pylori. In response to these challenges, quadruple therapy has emerged as a potent alternative. This regimen typically includes a PPI, bismuth salts (which possess intrinsic antimicrobial properties), and two antibiotics like tetracycline, metronidazole, or clarithromycin. Bismuth compounds help to suppress bacterial growth and mitigate resistance mechanisms, thereby improving treatment outcomes, especially in regions with high resistance rates. Sequential therapy represents another innovative approach in the H. pylori market. It involves administering a PPI and amoxicillin during the initial phase, followed by a PPI with clarithromycin and metronidazole in a subsequent phase. This sequential administration strategy leverages the varying susceptibility of H. pylori to antibiotics at different stages of treatment, potentially enhancing eradication rates compared to traditional triple therapies.
Moreover, tailored therapy guided by antibiotic susceptibility testing (AST) is gaining prominence. AST enables clinicians to identify the most effective antibiotics based on individual bacterial profiles, allowing for personalized treatment regimens. This approach not only optimizes eradication rates but also minimizes unnecessary antibiotic use, thereby reducing the development of further antibiotic resistance and improving patient outcomes. Overall, combination therapies in the H. pylori market demonstrate the evolving strategies to combat bacterial resistance and optimize treatment efficacy. As research continues to refine these approaches, the focus remains on achieving higher cure rates and reducing treatment failure, ensuring effective management of H. pylori infections worldwide.
Advances in Treatment Guidelines:
Recent advances in treatment guidelines for Helicobacter pylori infections have been shaped by evolving resistance patterns and the quest for improved therapeutic outcomes. Traditionally, standard therapies involved triple therapy with a proton pump inhibitor (PPI) and two antibiotics, typically clarithromycin and amoxicillin or metronidazole. However, increasing resistance to these antibiotics has prompted updates in treatment protocols. One significant advancement is the recommendation of quadruple therapy as a preferred first-line treatment in areas with high resistance rates. This regimen includes a PPI, bismuth salts, and two antibiotics like tetracycline and metronidazole or clarithromycin. Bismuth compounds exert antimicrobial effects and synergistically combat resistance mechanisms, leading to higher eradication rates compared to traditional triple therapy. Sequential therapy has also gained prominence as an alternative strategy supported by updated guidelines. This sequential approach involves initial treatment with a PPI and amoxicillin, followed by a subsequent phase with a PPI and clarithromycin plus metronidazole. Sequential therapy capitalizes on varying bacterial susceptibilities across treatment phases, potentially enhancing efficacy against resistant strains.
Furthermore, personalized medicine approaches, including antibiotic susceptibility testing (AST), are increasingly integrated into treatment guidelines. AST allows clinicians to tailor antibiotic selection based on individual bacterial resistance profiles, optimizing treatment efficacy and reducing the risk of treatment failure and further resistance development. Overall, these advances in treatment guidelines for the H. pylori market emphasize a shift towards more effective, adaptive treatment strategies. By leveraging quadruple and sequential therapies alongside personalized medicine principles, healthcare providers can enhance eradication rates, minimize treatment failures, and improve patient outcomes in managing H. pylori infections globally.
Buy Full Report: https://www.imarcgroup.com/checkout?id=8732&method=587
Top of Form
Bottom of Form
Leading Companies in the Helicobacter Pylori Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global helicobacter pylori market, several notable companies are actively engaged in developing novel therapies that target antibiotic-resistant strains of H. pylori. This includes exploring new antibiotics, combination therapies, and biopharmaceutical approaches to improve treatment outcomes. RedHill Biopharma and Phathom Pharmaceuticals have been investing heavily in their manufacturing capacities in recent months.
RedHill Biopharma Ltd. announced the issuance of a new U.S. patent covering Talicia as an all-in-one fixed-dose combination of amoxicillin, omeprazole, and rifabutin and its use for the treatment of Helicobacter pylori (H. pylori) infection (Patent No. 11,931,463, to be granted March 19, 2024).
Phathom Pharmaceuticals, on the other hand, disclosed that the FDA had approved reformulated vonoprazan tablets for Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin) to treat H. pylori infection in adults.
Apart from this, Takeda Pharmaceutical Company Limited, AstraZeneca K.K., Mitsubishi Tanabe Pharma Corporation, and Eisai Co., Ltd. announced that Japan's Ministry of Health, Labour, and Welfare has approved Helicobacter pylori gastritis as an additional indication for H. pylori eradication by triple therapy with proton pump inhibitors. This therapy includes a proton pump inhibitor, amoxicillin hydrate, and either clarithromycin or metronidazole.
Request for customization: https://www.imarcgroup.com/request?type=report&id=8732&flag=E
Regional Analysis:
The major markets for helicobacter pylori include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for helicobacter pylori while also representing the biggest market for its treatment. This can be attributed to the widespread adoption of alternative treatment regimens, such as quadruple therapy and sequential therapy, to improve eradication rates associated with this condition.
Moreover, the escalating shift towards more advanced diagnostic technologies for H. pylori detection in the U.S. is bolstering the market. This includes the adoption of rapid diagnostic tests, urea breath tests, and molecular diagnostics, which offer quicker and more accurate detection of H. pylori infections compared to traditional methods.
Apart from this, there is an increasing interest in personalized medicine approaches, such as AST, to tailor treatment regimens based on individual patient profiles. This trend aims to optimize treatment outcomes by selecting antibiotics that are most effective against the specific strain of H. pylori present in each patient.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the helicobacter pylori market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the helicobacter pylori market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current helicobacter pylori marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/helicobacter-pylori-market
IMARC Group Offer Other Reports:
Seizures Market: The 7 major seizures market reached a value of US$ 3.1 Billion in 2023, and projected the 7MM to reach US$ 4.9 Billion by 2034, exhibiting a growth rate (CAGR) of 4.17% during the forecast period from 2024 to 2034.
Cone Rod Dystrophy Market: The 7 major cone rod dystrophy market reached a value of US$ 114.5 Million in 2023, and projected the 7MM to reach US$ 179.0 Million by 2034, exhibiting a growth rate (CAGR) of 4.15% during the forecast period from 2024 to 2034.
Poliomyelitis Market: The 7 majors poliomyelitis market is expected to exhibit a CAGR of 5.04% during the forecast period from 2024 to 2034.
Genital Herpes Market: The 7 majors genital herpes market is expected to exhibit a CAGR of 3.49% during the forecast period from 2024 to 2034.
Somatotropin Deficiency Market: The 7 majors somatotropin deficiency market is expected to exhibit a CAGR of 3.99% during the forecast period from 2024 to 2034.
Viral Hepatitis Market: The 7 majors viral hepatitis market is expected to exhibit a CAGR of 2.69% during the forecast period from 2024 to 2034.
Cartilage Diseases Market: The 7 major cartilage diseases market reached a value of US$ 1.2 Billion in 2023, and projected the 7MM to reach US$ 3.9 Billion by 2034, exhibiting a growth rate (CAGR) of 11.64% during the forecast period from 2024 to 2034.
Deafness Market: The 7 major deafness market is expected to exhibit a CAGR of 3.3% during the forecast period from 2024 to 2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The market is driven by a shift towards non-invasive diagnostic methods, such as serological tests and stool antigen assays, reducing patient discomfort. Additionally, there is a growing focus on combination therapies involving proton pump inhibitors and antibiotics to improve treatment efficacy and reduce antibiotic resistance.
Rise of Non-Invasive Diagnostics: Driving the Helicobacter Pylori Market
The Helicobacter pylori (H. pylori) market has witnessed a notable rise in non-invasive diagnostic methods, marking a pivotal shift in how healthcare professionals diagnose and manage this prevalent bacterial infection. Numerous advancements in diagnostic technologies have introduced several non-invasive alternatives that are increasingly favored for their convenience, accuracy, and patient-friendly nature. One of the primary non-invasive diagnostic tools gaining prominence is the urea breath test (UBT). This test detects the presence of H. pylori by measuring the levels of carbon dioxide in the patient's breath after ingesting a urea solution. UBT is highly sensitive and specific, offering reliable results within a short timeframe, typically within 30 minutes to an hour. It is widely used in clinical settings and is considered a preferred method for diagnosing H. pylori due to its accuracy and non-invasive nature.
Request a PDF Sample Report: https://www.imarcgroup.com/helicobacter-pylori-market/requestsample
Additionally, serological tests have become another valuable non-invasive option. These tests detect antibodies against H. pylori in the patient's blood serum, indicating current or past infection. While serological tests are convenient and accessible, they are primarily used for screening purposes rather than for confirming active infection due to their inability to distinguish between past and current infections reliably. The adoption of non-invasive diagnostics in the H. pylori market is driven by several factors. Patients prefer these methods due to their minimal discomfort and avoidance of invasive procedures. Healthcare providers benefit from their efficiency and ease of use, enabling quicker diagnosis and initiation of appropriate treatment. Moreover, non-invasive tests are particularly valuable in primary care settings and resource-limited regions where access to specialized equipment and procedures may be limited. Moreover, the rise of non-invasive diagnostics represents a significant advancement in the management of H. pylori infections, improving patient care and streamlining healthcare practices. As technology continues to evolve, these methods are expected to play an increasingly critical role in early detection and effective treatment strategies for H. pylori, ultimately enhancing patient outcomes and reducing the overall burden of gastrointestinal diseases globally.
Combination Therapies: Contributing to Market Expansion
Combination therapies have revolutionized the treatment landscape for Helicobacter pylori infections, offering multifaceted approaches to overcome antibiotic resistance and enhance eradication rates. Traditionally, standard therapies involved triple combinations of proton pump inhibitors (PPIs) with antibiotics such as clarithromycin, amoxicillin, or metronidazole. However, the efficacy of these regimens has been increasingly challenged by the rise of antibiotic-resistant strains of H. pylori. In response to these challenges, quadruple therapy has emerged as a potent alternative. This regimen typically includes a PPI, bismuth salts (which possess intrinsic antimicrobial properties), and two antibiotics like tetracycline, metronidazole, or clarithromycin. Bismuth compounds help to suppress bacterial growth and mitigate resistance mechanisms, thereby improving treatment outcomes, especially in regions with high resistance rates. Sequential therapy represents another innovative approach in the H. pylori market. It involves administering a PPI and amoxicillin during the initial phase, followed by a PPI with clarithromycin and metronidazole in a subsequent phase. This sequential administration strategy leverages the varying susceptibility of H. pylori to antibiotics at different stages of treatment, potentially enhancing eradication rates compared to traditional triple therapies.
Moreover, tailored therapy guided by antibiotic susceptibility testing (AST) is gaining prominence. AST enables clinicians to identify the most effective antibiotics based on individual bacterial profiles, allowing for personalized treatment regimens. This approach not only optimizes eradication rates but also minimizes unnecessary antibiotic use, thereby reducing the development of further antibiotic resistance and improving patient outcomes. Overall, combination therapies in the H. pylori market demonstrate the evolving strategies to combat bacterial resistance and optimize treatment efficacy. As research continues to refine these approaches, the focus remains on achieving higher cure rates and reducing treatment failure, ensuring effective management of H. pylori infections worldwide.
Advances in Treatment Guidelines:
Recent advances in treatment guidelines for Helicobacter pylori infections have been shaped by evolving resistance patterns and the quest for improved therapeutic outcomes. Traditionally, standard therapies involved triple therapy with a proton pump inhibitor (PPI) and two antibiotics, typically clarithromycin and amoxicillin or metronidazole. However, increasing resistance to these antibiotics has prompted updates in treatment protocols. One significant advancement is the recommendation of quadruple therapy as a preferred first-line treatment in areas with high resistance rates. This regimen includes a PPI, bismuth salts, and two antibiotics like tetracycline and metronidazole or clarithromycin. Bismuth compounds exert antimicrobial effects and synergistically combat resistance mechanisms, leading to higher eradication rates compared to traditional triple therapy. Sequential therapy has also gained prominence as an alternative strategy supported by updated guidelines. This sequential approach involves initial treatment with a PPI and amoxicillin, followed by a subsequent phase with a PPI and clarithromycin plus metronidazole. Sequential therapy capitalizes on varying bacterial susceptibilities across treatment phases, potentially enhancing efficacy against resistant strains.
Furthermore, personalized medicine approaches, including antibiotic susceptibility testing (AST), are increasingly integrated into treatment guidelines. AST allows clinicians to tailor antibiotic selection based on individual bacterial resistance profiles, optimizing treatment efficacy and reducing the risk of treatment failure and further resistance development. Overall, these advances in treatment guidelines for the H. pylori market emphasize a shift towards more effective, adaptive treatment strategies. By leveraging quadruple and sequential therapies alongside personalized medicine principles, healthcare providers can enhance eradication rates, minimize treatment failures, and improve patient outcomes in managing H. pylori infections globally.
Buy Full Report: https://www.imarcgroup.com/checkout?id=8732&method=587
Top of Form
Bottom of Form
Leading Companies in the Helicobacter Pylori Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global helicobacter pylori market, several notable companies are actively engaged in developing novel therapies that target antibiotic-resistant strains of H. pylori. This includes exploring new antibiotics, combination therapies, and biopharmaceutical approaches to improve treatment outcomes. RedHill Biopharma and Phathom Pharmaceuticals have been investing heavily in their manufacturing capacities in recent months.
RedHill Biopharma Ltd. announced the issuance of a new U.S. patent covering Talicia as an all-in-one fixed-dose combination of amoxicillin, omeprazole, and rifabutin and its use for the treatment of Helicobacter pylori (H. pylori) infection (Patent No. 11,931,463, to be granted March 19, 2024).
Phathom Pharmaceuticals, on the other hand, disclosed that the FDA had approved reformulated vonoprazan tablets for Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin) to treat H. pylori infection in adults.
Apart from this, Takeda Pharmaceutical Company Limited, AstraZeneca K.K., Mitsubishi Tanabe Pharma Corporation, and Eisai Co., Ltd. announced that Japan's Ministry of Health, Labour, and Welfare has approved Helicobacter pylori gastritis as an additional indication for H. pylori eradication by triple therapy with proton pump inhibitors. This therapy includes a proton pump inhibitor, amoxicillin hydrate, and either clarithromycin or metronidazole.
Request for customization: https://www.imarcgroup.com/request?type=report&id=8732&flag=E
Regional Analysis:
The major markets for helicobacter pylori include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for helicobacter pylori while also representing the biggest market for its treatment. This can be attributed to the widespread adoption of alternative treatment regimens, such as quadruple therapy and sequential therapy, to improve eradication rates associated with this condition.
Moreover, the escalating shift towards more advanced diagnostic technologies for H. pylori detection in the U.S. is bolstering the market. This includes the adoption of rapid diagnostic tests, urea breath tests, and molecular diagnostics, which offer quicker and more accurate detection of H. pylori infections compared to traditional methods.
Apart from this, there is an increasing interest in personalized medicine approaches, such as AST, to tailor treatment regimens based on individual patient profiles. This trend aims to optimize treatment outcomes by selecting antibiotics that are most effective against the specific strain of H. pylori present in each patient.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the helicobacter pylori market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the helicobacter pylori market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current helicobacter pylori marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/helicobacter-pylori-market
IMARC Group Offer Other Reports:
Seizures Market: The 7 major seizures market reached a value of US$ 3.1 Billion in 2023, and projected the 7MM to reach US$ 4.9 Billion by 2034, exhibiting a growth rate (CAGR) of 4.17% during the forecast period from 2024 to 2034.
Cone Rod Dystrophy Market: The 7 major cone rod dystrophy market reached a value of US$ 114.5 Million in 2023, and projected the 7MM to reach US$ 179.0 Million by 2034, exhibiting a growth rate (CAGR) of 4.15% during the forecast period from 2024 to 2034.
Poliomyelitis Market: The 7 majors poliomyelitis market is expected to exhibit a CAGR of 5.04% during the forecast period from 2024 to 2034.
Genital Herpes Market: The 7 majors genital herpes market is expected to exhibit a CAGR of 3.49% during the forecast period from 2024 to 2034.
Somatotropin Deficiency Market: The 7 majors somatotropin deficiency market is expected to exhibit a CAGR of 3.99% during the forecast period from 2024 to 2034.
Viral Hepatitis Market: The 7 majors viral hepatitis market is expected to exhibit a CAGR of 2.69% during the forecast period from 2024 to 2034.
Cartilage Diseases Market: The 7 major cartilage diseases market reached a value of US$ 1.2 Billion in 2023, and projected the 7MM to reach US$ 3.9 Billion by 2034, exhibiting a growth rate (CAGR) of 11.64% during the forecast period from 2024 to 2034.
Deafness Market: The 7 major deafness market is expected to exhibit a CAGR of 3.3% during the forecast period from 2024 to 2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800