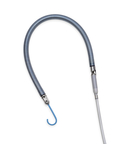
Abiomed (Nasdaq: ABMD) announces that Impella RP Flex with SmartAssist has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA), the FDA’s highest level of approval, as safe and effective to treat acute right heart failure for up to 14 days.
DANVERS, Mass.--(BUSINESS WIRE)-- Abiomed (Nasdaq: ABMD) announces that Impella RP Flex with SmartAssist has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA), the FDA’s highest level of approval, as safe and effective to treat acute right heart failure for up to 14 days. Impella RP Flex is implanted via the internal jugular (IJ) vein, which enables patient mobility, and has dual-sensor technology designed to optimize patient management.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221031005234/en/

Impella RP Flex with SmartAssist (Photo: Business Wire)
The Impella RP platform includes the world’s smallest percutaneous right heart mechanical circulatory support (MCS) technologies designed to help patients achieve native heart recovery. They do not require extracorporeal blood circulation and remain the only MCS technologies with FDA PMA indications for the treatment of right heart failure.
Key clinical benefits of Impella RP Flex include:
- Single venous access via the internal jugular (IJ) vein and 11 French (Fr) indwelling catheter, facilitating patient mobility
- Flexible cannula advanced over an extra-support guidewire, enabling ease of insertion and pump delivery
- SmartAssist dual-sensor technology with Impella Connect, providing advanced metrics to help with pump management and weaning
- Heparin-free purge, simplifying patient anticoagulant management with the use of sodium bicarbonate where heparin is of concern due to heparin intolerance or bleeding
“Impella RP Flex demonstrates Abiomed’s ongoing commitment to improving patient survival and achieving native heart recovery,” said Mark B. Anderson, MD, chairman of the department of cardiac surgery and cardiothoracic surgeon at the Heart and Vascular Hospital at HUMC/Hackensack Meridian Health.
Early identification of right heart failure or right ventricular dysfunction and early use of Impella RP is associated with significantly higher survival rates. Studies published in the Journal of Cardiac Failure and Journal of American College of Cardiology demonstrate that 37% of AMI cardiogenic shock (AMICS) patients exhibit right heart dysfunction (Lala et al.) and that right heart dysfunction is associated with three times increased risk of mortality (Mehta et al.). Data published in the Journal of Heart and Lung Transplantation shows patients who received Impella RP support within 48 hours of cardiogenic shock onset had a significantly higher survival rate than those who received delayed right-heart support (73% vs. 14%, p<0.001, Anderson et al.).
“The complexity of right ventricular failure has resulted in patients being underdiagnosed and undertreated,” said Robert Salazar, MD, an interventional cardiologist and director of cardiovascular research at Kingwood Medical Center. “Impella RP Flex is a novel tool that gives physicians the flexibility to treat this challenging patient population.”
The FDA indication for use of Impella RP Flex with SmartAssist is as follows:
The Impella RP Flex with SmartAssist System is indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥1.5 m2, who develop acute right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery.
Impella RP Flex will be introduced in the U.S. through a controlled rollout this quarter.
ABOUT IMPELLA RP WITH SMARTASSIST
Impella RP® with SmartAssist is U.S. FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant or open-heart surgery.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed (Nasdaq: ABMD) is a leading provider of medical technology that provides circulatory support and oxygenation. Our products are designed to enable the heart to rest and recover by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit abiomed.com.
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in Abiomed's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221031005234/en/
Contacts
Media:
Jenny Leary
Associate Director, U.S. Communications
+1 (978) 882-8491
jleary@abiomed.com
Investor:
Todd Trapp
Executive Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com
Source: Abiomed, Inc.