Indiana
Looking for an IT job? From data engineer to information security, check out the BioSpace list of 10 companies hiring life sciences professionals like you.
Looking for a research and development job? Check out the BioSpace list of 12 companies hiring life sciences professionals like you.
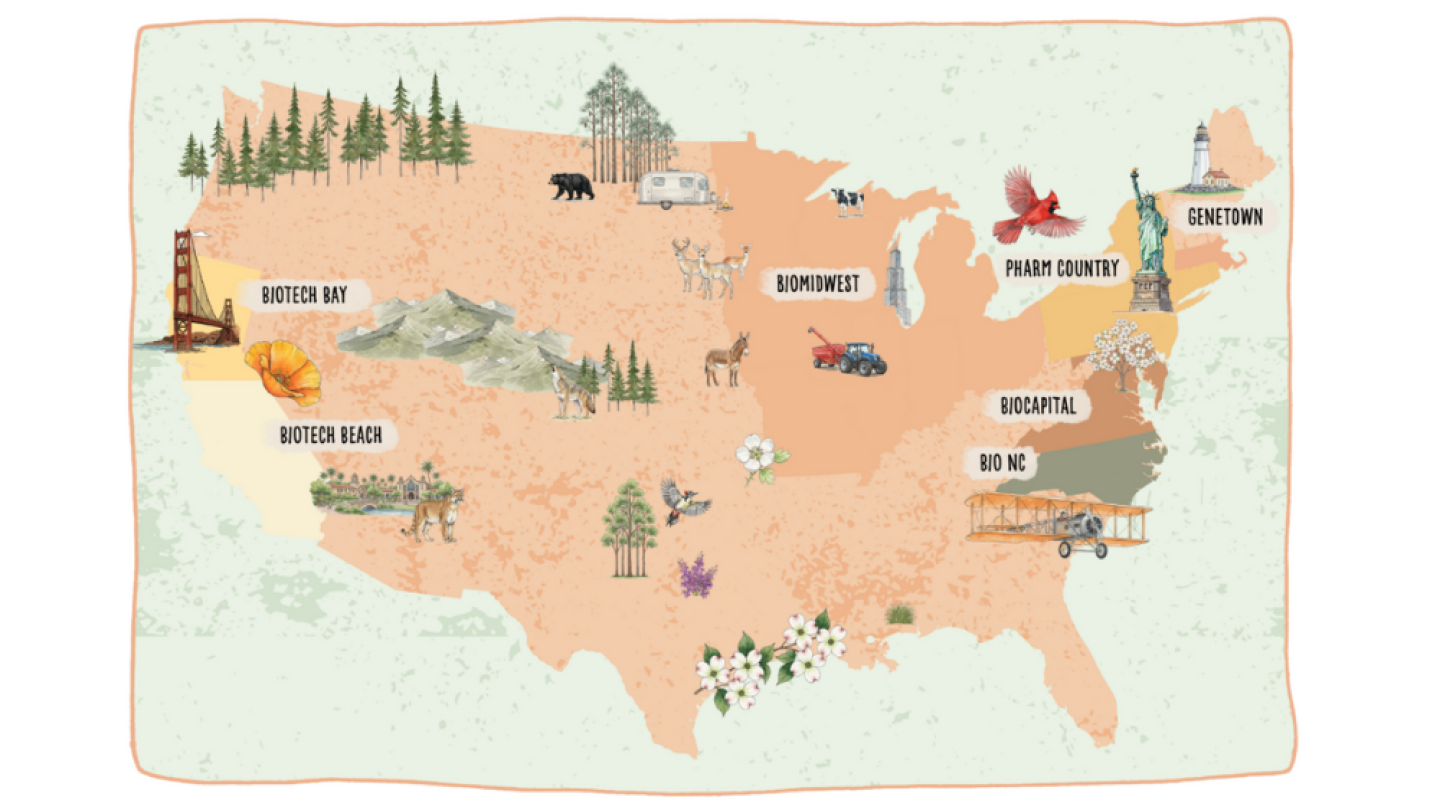
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
Following FDA rejections, Regeneron and Scholar Rock are turning to other facilities to clear regulatory logjams created by quality problems at an ex-Catalent facility in Indiana. Novo Nordisk, meanwhile, has been tight-lipped about whether its own FDA applications have been affected.
Looking for a job in regulatory? Check out the BioSpace list of eight companies hiring life sciences professionals like you.
The biopharma job market failed to turn around in May, but employers were still hiring, especially in Indiana and California, based on BioSpace data. The two states had the most job postings live on BioSpace last month, with Indiana showing a 108% year-over-year increase.
Looking for a manufacturing job? Check out the BioSpace list of 11 companies hiring life sciences professionals like you.
Major pharmaceutical companies are committing billions to US manufacturing in an effort to avoid steep tariffs threatened by President Donald Trump.
Companies are announcing significant investments in U.S. manufacturing in response to looming tariffs. An AstraZeneca executive and Eli Lilly and Novo Nordisk spokespeople discuss potential job and skill-building opportunities and where manufacturing might head in the future.
Looking for a biopharma job in Indiana? Check out the BioSpace list of seven companies hiring life sciences professionals like you.
PRESS RELEASES