Indica Labs, a leading provider of AI-powered digital pathology solutions, and Molecular Instruments®, the inventor of the HCR™ Imaging technology, announce a new partnership to co-market compatibility between MI’s patented HCR™ Imaging technology and Indica’s HALO® software.
ALBUQUERQUE, NM and LOS ANGELES, CA / ACCESSWIRE / April 5, 2024 / Indica Labs, a leading provider of AI-powered digital pathology solutions, and Molecular Instruments® (MI), the inventor of the HCR™ Imaging technology, announce a new partnership to co-market compatibility between MI's patented HCR™ Imaging technology and Indica's HALO® software.

HCR™ RNA-ISH sets a new industry standard with its array of clinical-grade features including mild sample preparation with no protease digestion, native integration with existing IHC/IF workflows, preserving tissue and cell/nuclear morphology for high quality image analysis. By using HALO® modules, such as ISH, FISH, ISH-IHC, and FISH-IF, researchers will be able to analyze both chromogenic and fluorescent HCR™ RNA-ISH images and perform fast, AI-based quantitative analysis on whole slide images.
Indica Labs' industry-leading HALO and HALO AI software enables high-throughput, AI-based quantitative tissue analysis. HALO modules simplify the analysis workflow so there is no need to ‘build' algorithms from scratch while generating expression data on a cell-by-cell basis. HALO Spatial Analysis and HALO AI deep learning classifiers are used in combination with any of the ISH modules to explore the distribution and spatial relationships between different cell phenotypes in the context of the tissue.
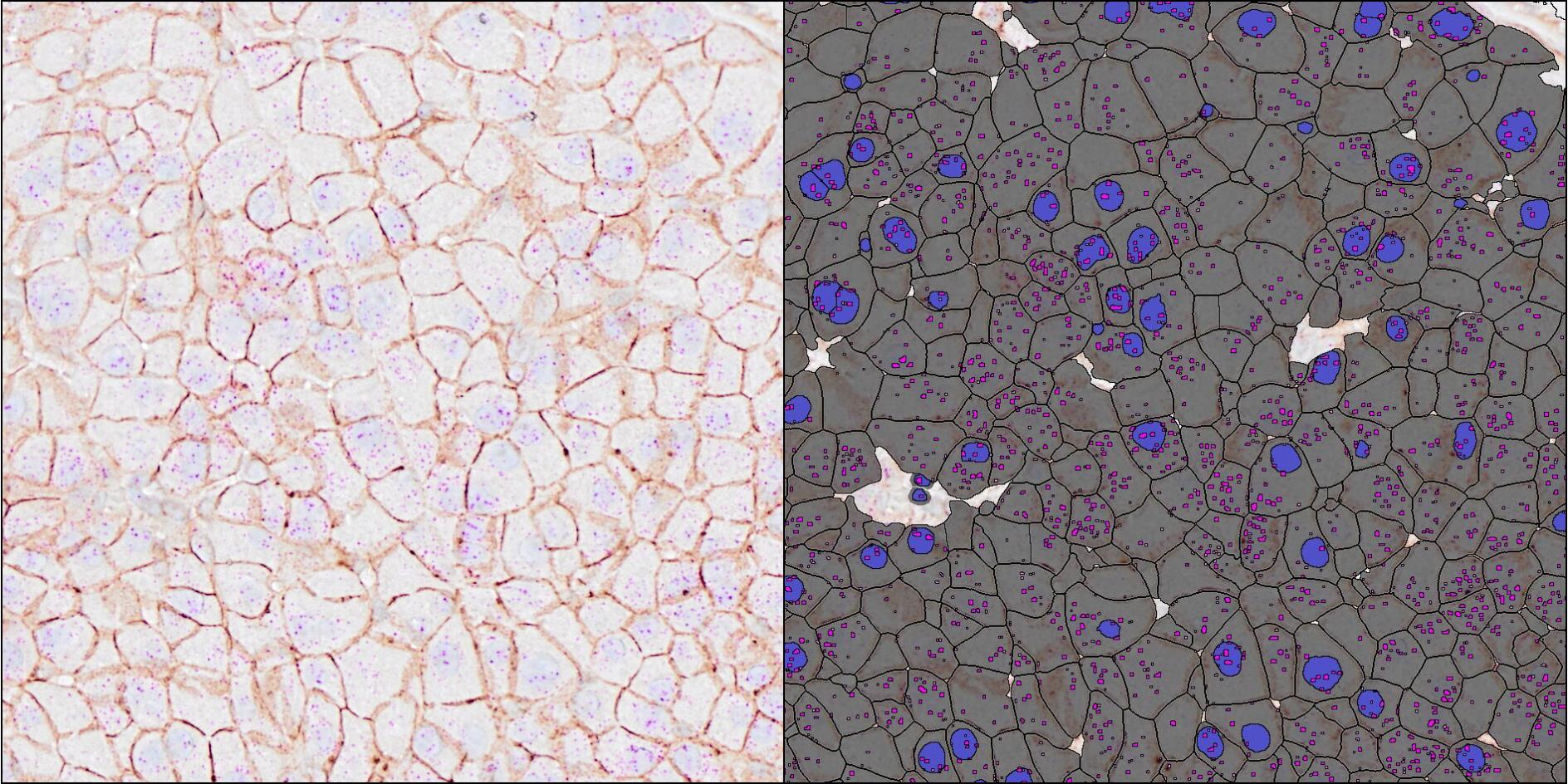
"We are excited to partner with MI to address the growing needs of our research customers for RNA-ISH assays that maintain cellular morphology," said Anne Hellebust, Director of Product, Life Sciences of Indica Labs. "Combining MI's HCR™ RNA-ISH technologies with our powerful AI-based analysis downstream enables us to provide a streamlined workflow for ISH staining and image analysis."
Dr. Aneesh Acharya, Chief Commercial Officer of Molecular Instruments, remarked "Building advanced tools that are accessible and easy to integrate into existing workflows is central to our mission at MI. Our partnership with Indica Labs symbolizes the dedication of both organizations to bringing state-of-the-art products to as many users as possible. We're thrilled that HCR™ Products are both compatible with all existing ISH analysis tools offered by HALO® and over time will enable novel analysis workflows previously considered impossible."
To learn more about the HCR™ Platform, please visit:
About Indica Labs
Indica Labs is the world's leading provider of AI-powered digital pathology software and services. Our flagship HALO® and HALO AI platform powers quantitative evaluation of digital pathology images. HALO Link facilitates research-focused image management and collaboration while HALO AP® delivers AI-powered diagnostics in a case-based platform. Through a commitment to open pathology and a combination of performance, scalability, and usability, our software solutions enable pharmaceutical companies, diagnostic labs, hospitals, research organizations, and Indica's own contract pharma services team to advance tissue-based research, clinical trials, and diagnostics.
About Molecular Instruments
Molecular Instruments® (MI) develops and synthesizes kits powered by its innovative HCR™ Platform for bioimaging applications in academic research, drug development, and clinical pathology and diagnostics. MI offers products for automated and manual chromogenic and fluorescence in situ hybridization (ISH) assays, featuring a protease-free workflow, native compatibility with existing IHC/IF assays, and straightforward image analysis. HCR™ Products are designed to be accessible to all researchers with complimentary introductory Starter Kits, free custom probe design, and pricing that makes sense.
Media contacts:
Indica Labs
Kate Lillard, PhD
kate@indicalab.com
Molecular Instruments
Joyce Yoo
joyce@molecularinstruments.com
SOURCE: Indica Labs
View the original press release on accesswire.com




