Today, Infinity BiologiX, LLC (IBX), Cytox Ltd, and Vanguard Pharma announce the launch of the IBX Alzheimer’s Risk Test, powered by genoSCORE™. Physicians are able to pre-register to order the test, which will be available from early October 2021.
New test provided through Infinity BiologiX (IBX) laboratories, powered by Cytox’s genoSCORE polygenic risk score platform, with Vanguard providing medical liaison and customer support available from early October 2021
The IBX Alzheimer’s Risk Test enables physicians to support patients with lifestyle changes, and guide use of drug therapy, to delay the onset of Alzheimer’s disease symptoms
For physician use only, the test can be ordered through the dedicated web portal: www.alzheimers-risk-test.com
PISCATAWAY, N.J. & OXFORD, England & MANCHESTER, England & MAHWAH, N.J.--(BUSINESS WIRE)-- Today, Infinity BiologiX, LLC (IBX), Cytox Ltd, and Vanguard Pharma announce the launch of the IBX Alzheimer’s Risk Test, powered by genoSCORE™. Physicians are able to pre-register to order the test, which will be available from early October 2021.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210921005715/en/
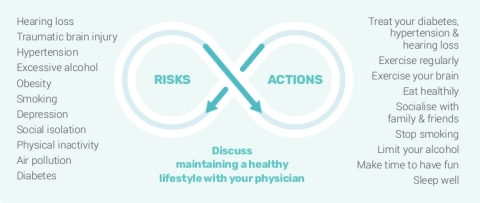
The following chart shows the well-documented modifiable risk factors(1) associated with Alzheimer’s disease, and executable actions that patients can take to mitigate the risks, with the potential to delay the onset of symptoms, and slow disease progression. (Graphic: Business Wire)
The new, non-invasive, test is the first physicians use only, clinically available and validated “polygenic” test, assessing over 100,000 genetic variations, to predict the risk of cognitive decline due to Alzheimer’s disease. The test only requires a simple saliva or blood sample, enabling individuals not wishing or easily able to attend healthcare settings, to provide a suitable sample from home.
Physicians can use the Alzheimer’s Risk Test to identify patients most at risk of cognitive decline, prior to invasive cerebrospinal fluid (CSF) testing or expensive scanning techniques. The results of the test can guide lifestyle changes and use of drug therapy to delay the onset of disease symptoms.
Dr. Richard Pither, CEO of Cytox, commented: “Modifiable factors account for more than 40% of the risk for dementia1. Consequently, it is important that clinicians confronted with patients with early cognitive concerns, have the appropriate tools to assist in understanding the future risk of disease progression to guide lifestyle advice and clinical management. This new test can support planning, by reducing the burden of additional testing in lower risk patients, and targeting monitoring and prevention approaches to those most likely to benefit.”
Robin Grimwood, IBX CEO, explained: “We are proud to partner with Cytox and Vanguard Pharma bringing the lab capabilities and expertise of the IBX team to bear on this cutting-edge solution. Enabling physicians to support their patients with the most clinically appropriate treatments is at the heart of what we do as an organization. This new test will provide physicians with a critical tool to stimulate lifestyle changes and therapy guidance prior to the onset of Alzheimer’s disease symptoms.”
Kevin Danylchuk, President of Vanguard Pharma added: “We are honored to be part of the launch of this exciting and innovative test. As part of the launch activities for the Alzheimer’s Risk Test, we will be hosting a number of different educational forums - both live and on-line that discuss how this test can be optimally used in clinical practice. Our aim is to ensure that the test is widely available to clinicians across the country who can use it to help guide patient needs and care decisions.”
About Infinity BiologiX (IBX) (www.ibx.bio)
IBX is a market-disrupting next-generation central laboratory supporting academia, government, and industry. IBX provides global sample collection, processing, storage, and analytical services integrated with scientific and technical support in both the research and clinical arenas. As a leader in biomaterials, IBX provides support to the development of diagnostics, therapeutics, and research in the genomics, precision, and regenerative medicine arenas. IBX previously operated as RUCDR Infinite Biologics before spinning off from Rutgers University-New Brunswick in August 2020.
About Cytox (www.cytoxgroup.com)
Without new drug therapies, the economic and healthcare cost burden of dementia - including Alzheimer’s disease (AD) - is estimated to exceed $1tn per year in the next decade2. AD – a highly complex disease with risk factors based in genetics, lifestyle, age, and environment. Cytox’s products genoSCORE™ and genoTOR™ use Polygenic Risk Scoring (PRS) to predict the risk of individuals developing AD, and to improve clinical trial outcomes through patient stratification and the genetic characterisation of the disease.
About Vanguard Pharma (www.vanguardpharma.com)
Vanguard Pharma is a leading healthcare contract sales organization serving the industry with innovative sales solutions, premier medical professional placement, and flexible partnerships that are required in today’s unpredictable marketplace. We offer unparalleled service to our clients and partners and exceptional support to our employees. In an industry that is facing many stresses and challenges, Vanguard Pharma remains committed to delivering quality solutions to meet the needs of both health care organizations and health care professionals as well as educators and sales staff alike.
About the IBX Alzheimer’s Risk Test, powered by genoSCORE™
genoSCORE is a new non-invasive, genetic test to predict future risk of cognitive decline due to Alzheimer’s disease (AD). The test, which uses a simple blood or saliva sample, analyses patient genotypes against an array of over 100,000 single nucleotide polymorphisms (SNPs) that are associated with AD. As such, the test generates a patient-specific polygenic risk score (PRS).
Clinicians can use the test to assess patients for the risk of developing Alzheimer’s, before symptoms arise, prior to invasive cerebrospinal fluid (CSF) testing or expensive scanning techniques. Furthermore, the test’s simplicity of use would enable elderly and vulnerable patients self-isolating due to COVID-19, or not wishing or easily able to attend healthcare settings, to provide a suitable sample from home.
Developers of new AD drug therapies can also the test to identify and recruit patients to clinical studies, ensuring the selection of the most suitable candidates, those most likely to experience cognitive decline over the time period of the study.
This test is a measure of relative risk for future onset of Late-onset Alzheimer’s disease (LOAD) and should not be considered as standalone diagnostic test. A high test score does not indicate that an individual will definitively develop LOAD in the future, and conversely a low test score does not categorically mean that subsequent onset of LOAD will never occur. The test result should be used in conjunction with other information available to the physician.
References:
- Lancet Commission: Dementia prevention, intervention, and care: 2020 https://pubmed.ncbi.nlm.nih.gov/32738937/
- World Alzheimer’s Report 2015 www.alz.co.uk/research/WorldAlzheimerReport2015.pdf
View source version on businesswire.com: https://www.businesswire.com/news/home/20210921005715/en/
Contacts
For physicians wanting to order the test please register at:
www.alzheimers-risk-test.com.
For physicians interested in hearing more about the test, please contact: alzheimers@vanguardpharma.com
Richard Pither, Chief Executive Officer, Cytox
Tel: +44 (0)1865 338 018
Adam Michael – Communications Lead, Media Contact, Cytox
Tel: +44 777 588 1813
adam.michael@cytoxgroup.com
Infinity BiologiX Media Contact:
Mike Thurogood mike.thurogood@infinity-biologix.com
Source: Infinity BiologiX, LLC
Smart Multimedia Gallery
The following chart shows the well-documented modifiable risk factors(1) associated with Alzheimer’s disease, and executable actions that patients can take to mitigate the risks, with the potential to delay the onset of symptoms, and slow disease progression. (Graphic: Business Wire)







