What is the best way to prevent or even reverse Alzheimer’s disease? That’s the question at the top of many researchers’ minds, especially given all of the recent large Phase 3 failures involving beta-amyloid targeting drugs, the most pursued target to date. This is troubling but not entirely shocking.
What is the best way to prevent or even reverse Alzheimer’s disease?
That’s the question at the top of many researchers’ minds, especially given all of the recent large Phase 3 failures involving beta-amyloid targeting drugs, the most pursued target to date. This is troubling but not entirely shocking given the historically enormous failure rate of 99.6 percent for Alzheimer’s drugs in development (only one of the 244 drugs tested in 413 clinical trials from 2002-2012 got FDA approved).
The plot just thickened when Biogen and Eisai recently announced they will pursue FDA approval for aducanumab, the anti-amyloid antibody drug they previously predicted would not hit its primary endpoints in the global Phase 3 trials ENGAGE and EMERGE and the Phase 2 trial EVOLVE, prompting the discontinuation of all three trials earlier this year. This dramatic reversal of their stance was triggered by rehashing the trial data and two meetings with the FDA. The biopharma industry continues to keenly watch and wait to see how this plays out.
In the wake of all this drama, we wanted to dive deeper and look more closely at what potential drug targets (especially non-amyloid targets) are being pursued to develop Alzheimer’s drugs. (For more information about the disease and a current overview of the drug pipeline, check out the recently published BioSpace Alzheimer’s report.)
Key Takeaways
- Alzheimer’s is not a part of normal aging.
- Beta-amyloid: Despite some setbacks with the discontinuation of large Phase 2 and 3 trials earlier this year, Biogen and Eisai are seeking FDA approval for their leading anti-amyloid antibody drug aducanumab.
- Tau: The presence and amount of tau tangles more closely correlate with cognitive impairment than the presence and amount of beta-amyloid plaques, giving hope that clearing tau tangles will provide a greater clinical benefit.
- Co-pathologies: Two other proteins (alpha-synuclein and TDP-43) have been found in the brains of Alzheimer’s patients in addition to beta-amyloid and tau.
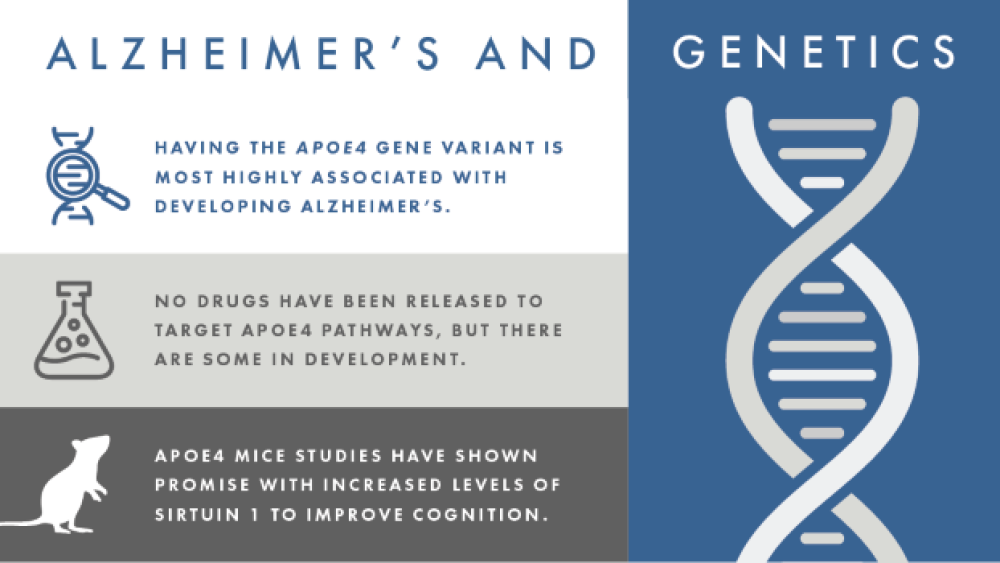
- Genetics: Having the APOE4 gene variant is most highly associated with developing Alzheimer’s disease, although not everyone who has the variant develops Alzheimer’s and not everyone with Alzheimer’s has the APOE4 variant.
- Neuroinflammation and microglial dysfunction: In Alzheimer’s, microglia (the brain’s housekeepers) don’t clear out waste, including beta-amyloid plaques, leading to neuroinflammation. However, we don’t fully understand why microglia don’t do their job.
- Mitochondrial dysfunction and oxidative stress: Oxidative stress, increased reactive oxygen species (ROS), and mitochondrial dysfunction all contribute to neuronal damage and death.
- Synapse loss: The aggregation of harmful proteins, other cellular debris, and chronic inflammation all contribute to neuron damage, synapse loss, neuronal death, and eventually the hallmark widespread atrophy of late-stage Alzheimer’s.
- Vascular changes: Changes in the brain’s blood vessels may cause decreased blood flow and breakdown of the blood-brain barrier, which increases inflammation and makes vascular problems worse, furthering this destructive positive feedback loop.
- Endosomal dysfunction: Enlarged endosomes have been reported to be the first change seen in Alzheimer’s brains.
- Brain hyperactivity/connectivity: Areas of decreased activity/connectivity in Alzheimer’s patients’ brains partially overlapped with the areas of hyperactivity/connectivity seen in young APOE4 carriers’ brains, indicating there may be a correlation (not necessarily causation) between the two.
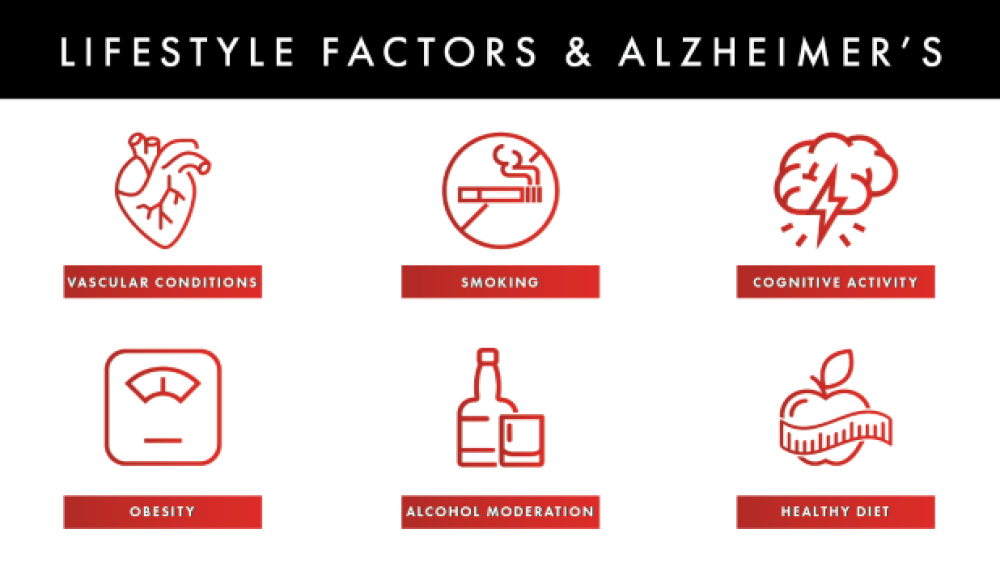
- Lifestyle factors: The link between cognitive decline, vascular conditions (high blood pressure, heart disease, stroke) and metabolic conditions (obesity and diabetes) is well known, but research is currently examining how these activities specifically impact the risk of cognitive decline and Alzheimer’s.
- The first randomized controlled trial, called the FINGER trial, showed that a multi-domain lifestyle intervention in older at-risk individuals can prevent cognitive decline. This model is now being studied in other countries, including the US (the US-POINTER trial).
What causes Alzheimer’s disease?
The cause of Alzheimer’s isn’t completely understood, but we know that it is a complex interaction between genetic, environmental, and lifestyle factors. Known risk factors include age (the number of people with Alzheimer’s doubles every 5 years after the age of 65), having a family history of Alzheimer’s disease, having a certain apolipoprotein E (APOE) gene called APOE4, having vascular problems (heart disease, stroke, high blood pressure), and having metabolic conditions (diabetes, obesity).
“Most likely, Alzheimer’s disease will turn out to be a cluster of diseases with slightly different initiators, accelerators, and exacerbators with no single cause or trigger,” Tetsu Maruyama, Chief Scientific Advisor of the Dementia Discovery Fund, told BioSpace.
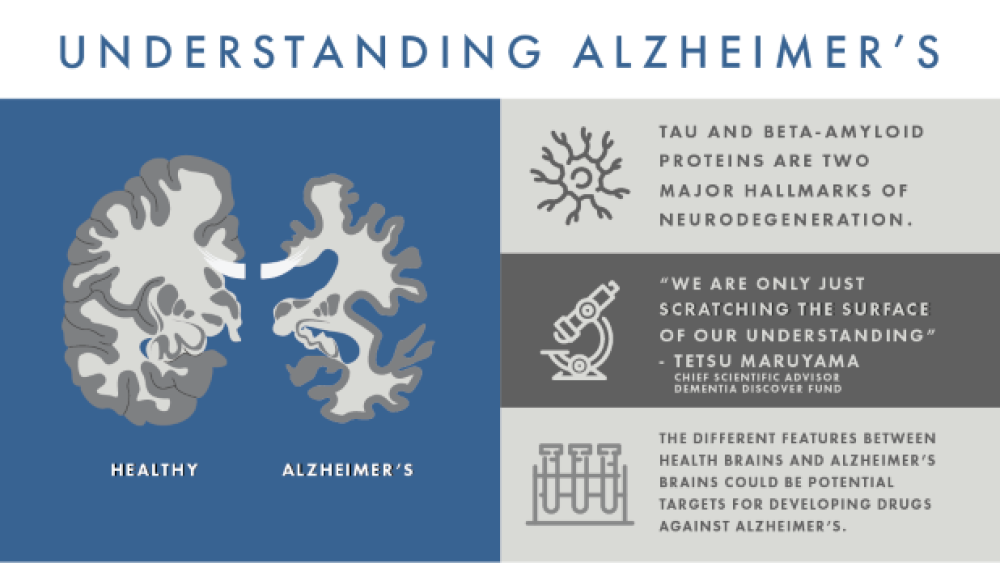
The uncertainty and complexity of what causes Alzheimer’s is largely to blame for the lack of successful drugs against the disease. Drug targets can only be identified when the differences between healthy and diseased cells are found, so understanding what is aberrant in Alzheimer’s brains, compared to normal aging brains, is the first step to identifying a target and developing an effective drug.
The aging brain versus the Alzheimer’s brain
Because Alzheimer’s is largely a disease of old age, studying normal brain aging may provide clues about the disease. Age-related changes include neuroinflammation, vascular damage, shrinking (atrophy) of certain brain areas, loss of cellular energy production (mitochondrial dysfunction) and production of reactive free radicals (harmful, highly reactive molecules).
As the brain ages, it is normal for it to shrink a bit, but it should not lose many neurons – that is one of the hallmark differences between normal aging and neurodegenerative diseases. In Alzheimer’s brains, neurons are destroyed, causing ever-increasing loss of connections between neurons, degrading communication and function. This manifests as the typical symptoms of memory loss, loss of reasoning and language skills, and abnormal social behavior.
Earlier efforts to find targets for Alzheimer’s focused on postmortem autopsies of Alzheimer’s patients to find molecular differences between their brains and healthy brains. Unusual deposits of two proteins, beta-amyloid (creating plaques) and tau (creating neurofibrillary tangles), were found in the Alzheimer’s brains.
“Two proteins – tau and beta-amyloid – are recognized as major hallmarks of neurodegeneration,” Andrea Pfeifer, CEO of AC Immune, told BioSpace. “Tangles and other abnormal forms of tau protein accumulate inside the brain cells and spread between cells, while plaques and oligomers formed by beta-amyloid occur outside the brain cells of people with Alzheimer’s.”
Other distinct features of the Alzheimer’s brain include chronic inflammation, vascular issues (any issue that affects the blood vessels, such as beta-amyloid plaque deposits in the arteries, hardening of the arteries (atherosclerosis), and mini-strokes), and loss of neuronal connections (ultimately leading to neuronal death).
“In my opinion, however, we are only just scratching the surface of our understanding,” Maruyama commented. “While all the ideas currently being tested have promise and should be pursued rigorously, we must also recognize how little we understand about the biology of neurons, microglia, astrocytes, oligodendrocytes and the contribution of vascular dysfunction to dementia.”
“The research we undertake in this field, no matter the institution, is all building up our knowledge of a disease that is one of the biggest threats to public health, and one of the biggest opportunities in healthcare,” Pfeifer added.
The features that differ between healthy and Alzheimer’s brains could be potential targets for developing drugs against Alzheimer’s.
Potential therapeutic targets
Based on the differences seen in Alzheimer’s brains, each potential cause is being pursued in the hopes of developing a drug that impacts the disease, from slowing to preventing to even reversing it.
Note: This is meant to give an overview of the therapeutic areas currently being explored in Alzheimer’s drug research. It may not include all ongoing research or potential targets.
Beta-amyloid plaques:
Beta-amyloid is a protein formed when a larger protein, aptly named amyloid precursor protein (APP), is broken down. There are multiple different forms of beta-amyloid, depending on the size of the fragment. Beta-amyloid is a naturally occurring protein in the brain and only wreaks havoc when it accumulates to abnormally high levels and begins to clump together (aggregate) between neurons. These protein aggregates, called plaques, disrupt the neuron’s function, prevents their communication to one another, and ultimately leads to their death.
While beta-amyloid is clearly seen in postmortem Alzheimer’s brains, it is believed to start accumulating decades before Alzheimer’s symptoms appear. This can now be seen using imaging molecules for positron emission tomography (PET) or magnetic resonance imaging (MRI) that light up beta-amyloid plaques in people living with Alzheimer’s, even those who aren’t currently symptomatic.
However, we still don’t know exactly how these plaques influence the symptoms of Alzheimer’s as there have been people with brains full of beta-amyloid plaques (seen postmortem) yet no outward cognitive symptoms. The amount of beta-amyloid seen in the brain postmortem also did not correlate with brain volume loss (brain atrophy).
Developing drugs against beta-amyloid plaques has been the primary focus of Alzheimer’s drug development. Unfortunately, we are starting to see that beta-amyloid alone may not be the best target for creating drugs to combat the disease.
“You will find airbags deployed at the scene of any serious automobile accident, but only in a small number of cases will the inappropriate deployment of the airbags have caused the accident, and simply looking at the vehicle afterwards will not tell you about cause and consequence,” Maruyama said. “So it would seem with amyloid in Alzheimer’s disease. Plaques are always there, but we don’t know how many are causative and how many are reactive.”
The research and funding focus are both beginning to shift away from beta-amyloid-targeting drugs. In fact, the Alzheimer’s Drug Discovery Foundation, a foundation supporting underfunded areas of preclinical and early-stage clinical research for Alzheimer’s disease, no longer funds anti-amyloid research.
Although the string of large Phase 2 and 3 trial failures earlier this year was thought to be the last nail in the beta-amyloid coffin, Eisai and Biogen stirred up the pot when they announced they were launching a Phase 3 trial (Clarity AD) for their anti-amyloid antibody drug BAN2401 in March, just one day after the major aducanumab trials were discontinued.
In a shocking turn of recent events, Biogen and Eisai’s announcement of pursuing FDA approval for aducanumab is shaking up the beta-amyloid theory once again. The companies said in a press release that “new analysis of [a] larger dataset showed that aducanumab reduced clinical decline in patients with early Alzheimer’s disease as measured by the pre-specified primary and secondary endpoints.” After re-analysis, the EMERGE trial and a subset of patients who received higher aducanumab doses in the ENGAGE trial met their primary endpoint of significantly reducing clinical decline. (Although the ENGAGE trial overall did not meet its primary endpoint.) Patients also reportedly showed significant improvements in memory, orientation, language, and daily living (doing chores, laundry, shopping, personal finance, etc.). If approved, aducanumab would be the first drug to show that removing beta-amyloid has a positive clinical impact and slows cognitive decline in Alzheimer’s. (Check out STAT’s Readout LOUD podcast for more perspective from experts, patients, and investors about Biogen’s aducanumab news.)
Tau tangles:
Tau is an intracellular protein that normally binds to and stabilizes microtubules (structures inside neurons that provide support and help transport nutrients throughout the cell). However, in Alzheimer’s, tau becomes detached from the microtubules and sticks to other tau proteins, forming long threads called neurofibrillary tangles (or tau tangles). These tangles prevent neurons from effectively transporting nutrients and other chemicals, which ultimately hurts communication between neurons (impacting memory function).
“Tau spreading in Alzheimer’s happens when there is concomitant presence of amyloid; if you can remove amyloid and stop tau from spreading, it may slow disease and potentially improve symptoms,” Pfeifer said. “Tau is the next most advanced potential therapeutic strategy beyond beta-amyloid, which may provide hope for effectively treating Alzheimer’s disease patients.”
Anti-tau therapies were initially focused on inhibiting kinases (ultimately tau phosphorylation) and tau aggregation or stabilizing microtubules. Unfortunately, studies of many of those potential drugs have been halted due to lack of efficacy or toxicity. Currently, tau immunotherapy is the major therapeutic focus and has shown potential by improving cognition in animal models.
The presence and amount of tau tangles more closely correlate with cognitive impairment than the presence and amount of beta-amyloid plaques. This provides hope that clearing tau tangles will provide a greater clinical benefit than the clinical disappointment seen with drugs that clear beta-amyloid plaques.
“In transgenic animals that overexpress tau, the tau antibody treatment does improve cognitive performance,” Pfeifer added. “Slowing the propagation of tau pathology may, therefore, slow disease progression and reduce cognitive decline, and anti-tau therapies have shown promise in slowing the progression of tau pathology in animal models of tauopathy.”
Co-pathologies (alpha-synuclein and TDP-43):
Two other proteins have been found in the brains of Alzheimer’s patients and associated with the disease: alpha-synuclein, a protein famous for its involvement with Parkinson’s disease; and transactive response DNA-binding protein of 43 kDa (TDP-43), a key protein involved with transcription that ultimately regulates protein production, especially involved with cellular clearance and autophagy pathways.
A recent study looked at the presence of various proteins, such as tau, beta-amyloid, alpha-synuclein, and TDP-43, in 766 autopsied patients with various neurodegenerative diseases, including 247 Alzheimer’s patients. They found that tau was in almost all (92-100 percent) and beta-amyloid was present in up to half (20-57 percent) of all neurodegenerative diseases. In the Alzheimer’s patients, both alpha-synuclein (41-55 percent) and TDP-43 (33-40 percent) were seen at higher percentages than in neurodegenerative diseases all together (4-16 percent with alpha-synuclein and 0-16 percent with TDP-43).
“It’s clear that Alzheimer’s disease is a multi-target disease with a high level of other proteinopathies and co-pathologies,” Pfeifer said. “These additional pathological proteins could play an important role in neurodegenerative diseases, including Alzheimer’s disease, and are an important part of our research.” (AC Immune’s pipeline is focusing on drugs against tau, beta-amyloid, alpha-synuclein, and TDP-43.)
In addition to its role in Parkinson’s, alpha-synuclein is also found in Alzheimer’s brains. It is thought to be involved with beta-amyloid plaque aggregation, although some studies did not find alpha-synuclein protein in beta-amyloid plaques via immunohistochemistry.
TDP-43 is also important in another type of dementia, called frontotemporal dementia, and amyotrophic lateral sclerosis (ALS, another neurodegenerative disease). In neurodegenerative diseases, TDP-43 is incorrectly localized to the cell’s cytoplasm, where it aggregates into insoluble clumps called inclusion bodies. TDP-43 inclusions have also been found in 19-57 percent of Alzheimer’s cases (analyzed via postmortem immunohistochemically stained brain samples). TDP-43 is thought to play a role in Alzheimer’s through both beta-amyloid-dependent and independent pathways. Although TDP-43 inclusions were also seen in postmortem brain samples of older people without any form of dementia, they were significantly more prevalent in postmortem brain samples from people with Alzheimer’s.
Genetics and epigenetics:
Having the APOE4 gene variant is most highly associated with developing Alzheimer’s disease, although not everyone who has the variant develops Alzheimer’s and not everyone with Alzheimer’s has the APOE4 variant. Although the APOE protein’s main job is to regulate blood cholesterol levels, it has since shown that the APOE4 protein is linked to beta-amyloid deposition and microglia activity against beta-amyloid plaques in a mouse model of plaque deposition. APOE4 was also seen to significantly enhance neurodegeneration in a mouse model of tauopathy (independent from its beta-amyloid effects). Having the APOE4 variant was also shown to increase risk of disease progression in symptomatic Alzheimer’s patients positive for beta-amyloid and tau deposits in their brains.
So far, there have been no drugs developed to target APOE4 pathways, but there are some in development. A 2016 paper was interested in targeting ATP binding cassette subfamily A member 1 (ABCA1), the main protein that adds fat molecules (lipidates) APOE. Because APOE4 is hypolipidated, increasing its lipidation is thought to help increase its function. A peptide that activates ABCA1(called CS-6253) was shown to increase APOE4 lipidation, which reversed the APOE4-driven beta-amyloid plaque accumulation and tau hyperphosphorylation in the hippocampal neurons in a mouse model.
A 2018 paper looked at how a small molecule (called A03) impacted levels of sirtuin 1 (SIRT1), a neuroprotective enzyme, in the brains of mice with the APOE4 gene. The mice treated with A03 had increased sirtuin 1 expression in their hippocampi and improved cognition. APOE4 is known to decrease sirtuin 1 levels, which has been linked to increased tau acetylation (which happens in Alzheimer’s and other tauopathy brains).
Another interesting idea is using small molecule “structure correctors” to modify the structure of APOE4 and prevent its harmful effects. The APOE4 protein has an altered structure that makes it much more susceptible to being cut up into neurotoxic fragments (via proteolytic cleavage). One 2018 paper showed that a small molecule structure corrector (called PH002) reduced the neurotoxic effects of APOE4 via reduction of tau phosphorylation, beta-amyloid production, and neuron degeneration in stem cell-derived human neurons.
Chronic neuroinflammation (microglia regulation):
Inflammation in the brain is thought to be caused by the buildup of glial cells (cells that support the neurons) and cellular waste. Specific glial cells called microglia (sometimes called the macrophages of the brain) engulf cellular waste and rid the brain of those toxins, acting as the brain’s housekeepers. In Alzheimer’s, microglia don’t clear out waste, including beta-amyloid plaques, leading to neuroinflammation. The link between this abnormal microglia activity and Alzheimer’s progression is well established, but why they fail to function properly is still being teased apart.
One possible target for microglia function is triggering receptor expressed on myeloid cells 2 (TREM2), a gene expressed on microglia that may instruct them to get rid of dead neurons and beta-amyloid plaques from the brain, ultimately decreasing inflammation. Interestingly, APOE (among other apolipoproteins) can bind to the TREM2 protein and activate it. According to the National Institute on Aging, people who have a dysfunctional TREM2 gene have plaque buildup between neurons and accumulated non-functional microglia and astrocytes (another type of glial cell) around neurons, which causes chronic inflammation and further neuronal damage. Previous papers have linked TREM2 variants with increased risk of Alzheimer’s due to increased neuroinflammation and a build-up of beta-amyloid plaques. Therefore, TREM2 is thought to protect the brain from Alzheimer’s by altering beta-amyloid compaction and preventing neuronal damage.
However, a 2017 paper showed the opposite effect in mice that form tau tangles in their brains (called a tauopathy mouse model). Nine-month-old mice that lacked the TREM2 gene had significantly less brain atrophy and reduced microglial activation (reduced microgliosis) in the hippocampus compared to mice with the TREM2 genes. Therefore, for pure tauopathies, lacking TREM2 may provide protection against neurodegeneration by reducing neuroinflammation. The authors discussed this unexpected result and suggested that TREM2 expression may play dual roles in beta-amyloid versus tau pathologies. It is also important to note this was one experiment on a mouse model, so the results need to be replicated and studied in humans.
A 2015 review paper also discussed other genes that may increase the risk for Alzheimer’s, including complement receptor 1 (CR1), cluster of differentiation 33 (CD33), and toll-like receptor 4 (TLR4). The authors noted that Alzheimer’s risk associated with CD33 and TLR4 function were conflicting, indicating how complex neuroinflammation is in relation to Alzheimer’s risk. Another 2018 review noted the connection between neuroinflammation and the CX3C chemokine receptor 1 (cx3cr1) and progranulin pathways, which both keep the microglial inflammatory response in check, among other functions. Progranulin has also been found to be involved with frontotemporal dementia.
A recent paper showed that microglia-mediated damage was the main cause of neurodegeneration, not direct tau-induced neurotoxicity, in a tauopathy mouse model expressing a mutant form of human tau. This study helped tease apart whether the microglia or tau tangles were primarily contributing to neurotoxicity and ultimately neurodegeneration. They also noted that APOE largely affected microglia activity and had a minor role in regulating tau tangles.
Oxidative damage:
During normal cell metabolism, reactive molecules are produced as byproducts called reactive oxygen species (ROS). Over time, ROS can build up and aberrantly react with other molecules and cells, causing damage and, ultimately, contributing to cellular aging and cell death. The brain is particularly sensitive to ROS and oxidative stress because it is so metabolically active and, therefore, has a high oxygen consumption.
Oxidative stress and ROS contribute to synapse dysfunction and neuronal death, but how exactly they do that is not well understood. Oxidative stress has also been shown to mediate abnormal tau hyperphosphorylation, which could contribute to Alzheimer’s progression. Despite increasing evidence linking ROS and the development of Alzheimer’s, antioxidant therapies have yet to show consistent results in clinical trials with only a few trials showing the slowing of cognitive decline. For example, in five clinical trials looking at the effect of vitamin E (alone or in combination with other drugs), an antioxidant supplement, only one reported slowing of Alzheimer’s progression (the other four reported no effect on cognitive function).
Because oxidative stress, antioxidants, and diet are so closely related, there are also studies looking into nutritional interventions of how dietary habits affect oxidative stress and neurodegenerative diseases. (There is more about diet and Alzheimer’s risk under the “Lifestyle factors” section later in this report.)
Mitochondrial dysfunction:
Mitochondria are not only the main source of ROS in cells, but they are also the main cellular energy producers. If they go awry, cells can’t get the energy they need to function properly (due to defective glucose metabolism by mitochondria) and may die off. Mitochondrial dysfunction has been identified as an early event in Alzheimer’s due to the reduced brain cell metabolism, reduced synaptic functioning, increased ROS production, and increased brain cell death.
There is currently debate as to how mitochondrial dysfunction and beta-amyloid plaque formation are related – like the chicken and the egg problem. Some believe that the beta-amyloid plaques cause mitochondria to not function properly, while others argue that mitochondrial dysfunction occurs before plaque aggregation and may even play a role in their formation. Other data indicate that mitochondrial dysfunction and plaque formation are independent of one another.
One possible way to modulate mitochondria function is through the voltage-dependent anion channel 1 (VDAC1) protein. VDAC1 is a major part of the mitochondrial membrane that acts as a channel to let ions and other chemicals in and out of the mitochondria. This regulates many metabolic and energetic mitochondrial functions, including oxidative stress, mitochondrial-mediated apoptosis, and calcium regulation. VDAC1 also interacts with many other proteins, including beta-amyloid and phosphorylated tau. Postmortem analysis of Alzheimer’s patients’ brains reveals high levels of VDAC1; VDAC1 overexpression is known to trigger cell death, so targeting VDAC1 to regulate mitochondrial dysfunction may curb brain cell death.
Other possible targets to regulate mitochondrial function are PTEN-induced putative kinase 1 (PINK1), which is important in removing damaged mitochondria and was shown to be less expressed in Alzheimer’s brain samples, and sirtuin 3 (SIRT3), which is essential to many mitochondrial functions and was shown to have significantly decreased expression in the cortex of postmortem Alzheimer’s brain samples.
The sigma-1 and muscarinic receptors have also been implicated in neurodegenerative diseases by acting as general modulators of many cellular mechanisms, including microglial regulation, mitochondrial dysfunction, oxidative damage, and endoplasmic reticulum stress. A mixed sigma-1/muscarinic receptor antagonist (ANAVEX 2-73) has been shown to prevent mitochondrial dysfunction (and the resulting oxidative damage) and reduce tau hyperphosphorylation and beta-amyloid accumulation in the brains of Alzheimer’s mouse models.
Neuronal connection (synapse) loss:
The aggregation of harmful proteins, other cellular debris, and chronic inflammation all contribute to neuron damage and eventually death. As more and more neurons die off, connections between neurons (called synapses) get broken. Regions of the brain where neurons are dying shrink, causing brain atrophy; widespread brain atrophy is a hallmark of late-stage Alzheimer’s. Synapse loss and brain atrophy are best correlated with cognitive decline, making understanding how to control and prevent excess synapse loss important for developing potential therapeutics.
Microglia may not only be important in neuroinflammation, but also in synapse loss. Microglia engulf synapses to remove them (via phagocytosis), contributing to synaptic pruning during development and throughout adulthood. This is particularly important for drug development because overstimulating microglia activity to clear beta-amyloid plaques needs to be balanced with microglia-mediated synapse loss; in other words, drugs that stimulate microglia activity need to be careful not to overstimulate them and trigger synapse loss.
The most understood regulator of microglia-mediated synapse loss is through specific parts of the complement system, a part of the innate immune system that enhances (complements) antibodies and other parts of the immune system to help fight off microbes. A 2016 paper showed that inhibiting certain molecules in the pathway (C1q, C3, and CR3) decreased the number of phagocytic microglia, thereby curbing synapse loss in an Alzheimer’s mouse model. The authors suggested that this meant that microglia and the complement pathway are “inappropriately activated” in Alzheimer’s, leading to synapse loss.
Vascular changes:
Changes in the brain’s blood vessels may cause decreased blood flow and inadequate blood supply (and therefore oxygen) to the brain and may even contribute to breakdown of the blood-brain barrier. This increases inflammation, which makes vascular problems worse, furthering this destructive positive feedback loop. Disrupting this loop may both help prevent Alzheimer’s and further vascular damage in the brain. A recent review article discussed this vicious cycle and how the vascular component of Alzheimer’s is poorly explored thus far.
Neuroimaging techniques, such as certain kinds of magnetic resonance imaging (MRI), can be used to visualize blood flow in different areas in the brain. This is useful for not only seeing the differences between normal and Alzheimer’s brains, but also for looking for early changes in blood flow that could act as predictors of Alzheimer’s disease prior to the onset of symptoms.
One interesting application of vascular changes from Alzheimer’s is the possibility of using a non-invasive eye test to look for the disease. Studying an interesting pair of 96-year-old identical twins (one with Alzheimer’s and one without), researchers saw significantly reduced blood vessel density in the eyes of the twin with Alzheimer’s compared to the eyes of the cognitively healthy twin. This prompted the researchers to study more than 200 people to see if these differences could be distinguished in a larger population. The 39 Alzheimer’s patients did, in fact, have less dense blood vessels in their eye compared to both 133 healthy patients and even 37 mild cognitive impairment (MCI) patients. The authors suggest the vascular changes seen in the eye may mirror vascular changes that happen in the brain in Alzheimer’s patients.
Another recent study looked at the difference between 29 PET scan-confirmed amyloid-positive Alzheimer’s patients, 28 amyloid-negative subcortical vascular cognitive impairment (SVCI) patients, and 34 cognitively healthy patients. They reported no significant differences in blood vessel density and complexity (retinal fractal dimension) between the amyloid-positive Alzheimer’s patients and cognitively healthy patients. The amyloid-negative SVCI patients did have less dense and complex blood vessels in their eye (smaller total and arteriolar fractal dimensions) compared to cognitively healthy patients.
Enlarged endosomes and endosomal abnormalities:
Endosomes are membrane “bubbles” that transport nutrients and other molecules into and around the cell. They are formed when the cell’s membrane folds inward, wraps around the molecules, and pinches off from the membrane, forming its own circular structure. Endosomes typically bring the molecules to the lysosome (another internal structure in the cell) for digestion.
Disruption in this endosomal-lysosomal pathway is known to happen in Alzheimer’s. Enlarged endosomes have been reported to be the first change seen in Alzheimer’s brains. Endosomal/lysosomal dysfunction has also been implicated prior to and simultaneously with beta-amyloid accumulation; genes known to promote amyloid plaque formation, such as APOE4, APP, and presenilin-1 and -2 (PSEN1/2), also affect the endosomal-lysosomal pathway.
These dysfunctional endosomes or lysosomes cause cellular “traffic jams” as they cannot properly shuttle molecules around the cell or degrade molecules, backing up waste products like beta-amyloid. This contributes to the build-up of toxic proteins in and between brain cells seen in Alzheimer’s.
“Enlarged endosomes are a well-known but often overlooked pathological feature of Alzheimer’s disease, and ideas about the role endosomal and/or lysosomal dysfunction might contribute to Alzheimer’s are also advancing,” Maruyama said.
Two recent papers both focus on sodium/hydrogen exchanger 6 (NHE6), an enzyme located in the endosome membrane that shuttles ions across the membrane to help acidify the inside of the endosome (which facilitates the breakdown of molecules inside). One paper showed that NHE6 is downregulated in APOE4-containing cells, which causes ineffective clearing of beta-amyloid. Increasing NHE6 to normal levels via epigenetic modification corrects beta-amyloid clearance. However, the other paper reported the opposite; inhibiting NHE6 (both with drugs and genetic methods) reverses the APOE4-linked endosomal processing blockage.
Hyperactivity and hyperconnectivity:
There was a very interesting paper published recently connecting the dots between the brain activities of healthy young APOE4 carriers and people with Alzheimer’s. Resting-state brain activity in 108 young healthy non-APOE4 carriers (18-65 years old, average age of 24) was compared to that of 51 young people with at least one copy of APOE4. The young APOE4 carriers showed increased brain activity and connectivity in areas of the right side of the brain, which are usually active when someone is not focusing on a task (called the default mode network).
The same researchers previously saw decreased neuronal activity and connectivity in the brains of people with early-stage Alzheimer’s compared to age-matched controls without Alzheimer’s. The interesting part is that the areas of decreased activity/connectivity in Alzheimer’s patients’ brains partially overlapped with the areas of hyperactivity/connectivity in the young APOE4 carriers. The authors suggest that “brain areas that end up getting impaired in Alzheimer’s may be highly active and connected early in life – long before symptoms of the disease appear.” It is important to note that this study is an observational study, so it only finds a correlation between brain activities and cannot say anything about a causative relationship.
Lifestyle factors:
Studying the impact of lifestyle on Alzheimer’s onset and symptoms is a hot topic to research right now. The link between cognitive decline, vascular conditions (high blood pressure, heart disease, stroke) and metabolic conditions (obesity and diabetes) is well known. However, understanding how these activities specifically impact the risk of cognitive decline and Alzheimer’s is important because they can be immediately implemented and tend to have fewer side effects than the available Alzheimer’s drugs.
“Until we can identify a treatment or cure, lifestyle factors such as exercising, eating a healthy diet, keeping your brain active, reducing risk factors for cardiovascular disease (high blood pressure, cholesterol, diabetes, and smoking), and getting enough high-quality sleep are beneficial,” Pfeifer commented.
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) is the “first randomized controlled trial showing that it is possible to prevent cognitive decline using a multi-domain lifestyle intervention among older at-risk individuals,” according to the Alzheimer’s Association. The FINGER trial studied the effects of interventions such as physical exercise, nutritional guidance, cognitive training, social activities, and managing vascular risk factors on 1,260 adults aged 60-77 who had a cognitive performance at or slightly lower than the mean value for their age. Cognition was assessed two and seven years after enrolling. The 2-year follow-up (finalized in February 2014) showed that participants in the intervention group had 25 percent higher cognitive scores than those in the control group.
“The results of the FINGER trial highlight the value of addressing multiple dementia risk factors as a strategy to protect brain health and promote overall health and functioning,” Pfeifer said.
The FINGER model is being tested in other countries, including the United States, China, Singapore, and Australia, referred to as “World Wide FINGERS (WW-FINGERS).” The United Stated adaption called the U.S. Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (US-POINTER), is ongoing and currently enrolling at some sites. To see if you or someone you know qualifies, check out the study’s information.
A recent paper published in JAMA showed that a healthy lifestyle (as defined by factors such as not smoking, regular physical activity, healthy diet, and moderate alcohol consumption) is associated with a lower risk of developing dementia regardless of genetic risk (as defined by a polygenetic risk score including multiple common variants associated with Alzheimer’s and dementia). This retrospective study included almost 200,000 people of European descent ages 60 and older without dementia at the study’s start who joined the UK Biobank study from 2006-2010 and were followed-up until 2017.
UsAgainstAlzheimer’s also recently published a review report on non-pharmacological therapies for Alzheimer’s. The foundation states that non-pharmacological therapies, such as diet, exercise, cognitive training, or medical devices, are promising, but underexplored and underfunded.
Diet
Eating a healthy, balanced diet should be a priority for everyone, but what exactly does a “healthy” diet look like to help prevent cognitive decline (if diet can even impact cognition)? The Alzheimer’s Association recommends a low saturated fat, high unsaturated fat diet rich in fruits and vegetables, such as the Mediterranean or Dietary Approaches to Stop Hypertension (DASH) diets (which both also have the benefits of lowering your risk of cardiovascular disease and losing weight).
A 2018 study conducted by Lisa Mosconi and her lab looked at the differences in brain scans and biomarkers over a 2 (or more)-year period in people who did (34 people) and did not (36 people) follow the Mediterranean diet. Participants ranged from 30 to 60 years old and did not show signs of dementia at the start of the study. Brain scans at the beginning of the study already showed fewer beta-amyloid plaques and more glucose uptake in the brain (generally corresponding to brain activity) in people who followed the Mediterranean diet compared to those that did not. By the second scan at least 2 years later, the differences in plaques deposits and glucose usage were even more pronounced between the groups. While this study was relatively small and included mostly white female participants, it suggests our diets have a direct impact on brain function, even before we have any cognitive symptoms. This is important because diet is something we can easily control, something we can actively do to delay a disease with no cure currently.
A 2011 study in 2,258 older people (mean age of 77) without dementia found that higher adherence to the Mediterranean diet (as assessed on a nine-point scale) was associated with a lower risk of having Alzheimer’s (as determined through multiple cognitive tests) at a 1.5-year follow-up. Interestingly, the 262 people that developed Alzheimer’s during the study had significantly lower adherence to the Mediterranean diet, were older, less educated, and had a lower body mass. Also, a significantly higher portion of Hispanic people developed Alzheimer’s.
A 2014 review article showed that higher adherence to the Mediterranean diet was associated with a lower risk of Alzheimer’s and mild cognitive impairment (MCI). A 2016 review article showed that high adherence to the Mediterranean, DASH, and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets were all associated with reduced risk of developing Alzheimer’s. The DASH and MIND diets are similar to the Mediterranean diet (plant-based foods, limited saturated fat and meat consumption) and vary in terms of specific vegetable, fruit, dairy, fish, and potato consumption recommendations.
A 2016 review article examined 32 studies (5 randomized control trials and 27 observational studies) that looked at the relationship between the Mediterranean diet and cognition (cognitive function, cognitive impairment, dementia, or Alzheimer’s). Of those studies, most showed an association between the Mediterranean diet, improved cognitive function, and decreased risk of cognitive impairment, dementia, and Alzheimer’s. However, some studies showed no correlation between the Mediterranean diet and cognitive function (5 studies), cognitive impairment (3 studies) or Alzheimer’s (3 studies). The authors also made the important distinction that most of these studies provided evidence for correlation and not a cause-and-effect relationship.
Exercise
Staying physically active has not only a positive impact on your body but also helps support a healthy mind. The Alzheimer’s Association recommends regular cardiovascular exercise that increases your heart rate (colloquially called “cardio”) because it increases blood flow throughout your body, including your brain.
Exercise has multiple positive effects on brain health by increasing blood flow (and therefore nutrients) to the brain, enhancing the creation of new neurons (neurogenesis), increasing hippocampal volume, decreasing oxidative stress and ROS, and reducing the amount of beta-amyloid plaques and tau tangles. People who use exercise to treat Alzheimer’s show improved cognition, a slower decline in daily activities, and decreased psychiatric symptoms (confusion, psychosis, hallucinations, etc.).
Exercise not only positively impacts the brain, but it also lowers the chance of having risk factors for Alzheimer’s disease, such as high blood pressure, diabetes and high cholesterol. Exercise-induced improvement of cardiovascular fitness was also associated with improved memory and decreased hippocampal atrophy in a randomized controlled trial in 68 older adults with probable Alzheimer’s. The participants either did 150 minutes per week of aerobic exercise or non-aerobic stretching and toning for 26 weeks. The authors suggested that improved cardiovascular symptoms may be important for “driving brain benefits.”
It is important to note that conducting robust exercise clinical studies are difficult and more studies are needed to fully understand if and how exercise is enhancing cognition or staving off Alzheimer’s. (Although, it’s not a bad idea to adopt regular exercise habits for your overall health anyway.)
Cognitive stimulation
Working out isn’t limited to the body – “cognitive exercise” can be just as important. Keeping your mind engaged and challenged throughout your life can be beneficial to your brain health. The Alzheimer’s Association suggests getting as much formal education as possible, learning a new skill, adopting a new hobby, engaging in a challenging game or reading high-level material are all good ways to stay mentally active.
Cognitive exercise can be broken down into three categories: cognitive training (repeated exercises to achieve specific cognitive function goals, such as better frustration tolerance and problem-solving skills), cognitive stimulation (puzzles, word games, and indoor gardening), and cognitive rehabilitation (reasoning exercises).
A recent randomized controlled trial looked at how cognitive training twice a week for six months (compared to no training) impacted cognition in Alzheimer’s patients. The 45 patients who received cognitive training scored significantly better on cognitive tests right after completing the six months of training whereas the 85 patients who received no training scored significantly worse. This trend was true even six months after training ended; the trained group’s test scores “remained stable” while the untrained group’s scores got worse, indicating progressive cognitive decline over time. The authors suggested that cognitive training may “improve cognitive functions” in Alzheimer’s patients and “temporarily slow their cognitive decline.”
Music therapy is another non-pharmacological option that has been widely adopted to treat both cognitive and behavioral symptoms in Alzheimer’s patients. A recent review article reported that listening to music may help the patient relax, providing long-term benefits, and listening to individualized music had the best effects on the patient. Active music therapy provides more immediate benefits of a cognitive challenge and social interaction.
Social activity
Regular social interaction is associated with reduced risk of depression and improved brain health, according to the Alzheimer’s Association. In fact, previous studies showed a correlation between loneliness and risk of developing Alzheimer’s – lonely people had a higher risk of developing Alzheimer’s than non-lonely people. Engaging with family and friends, participating in clubs or hobby classes, and volunteering are all wonderful ways to stay socially engaged.
One idea of why social interaction may aid cognition involves boosting brain-derived neurotrophic factors (BDNF), which promotes neuron survival and is involved in their growth, maturation, and maintenance. Decreased BDNF contributes to cognitive decline seen in aging and Alzheimer’s. In an Alzheimer’s disease mouse model, mice that were kept in the same cage as other mice (co-housed) showed improved memory and increased BDNF expression compared to mice housed alone.
One small study suggested that social interaction with a familiar person may help Alzheimer’s patients learn better due to using different ways of understanding the information (different neural resources) through social interaction. Another study noted the social interactions, conversations, and sharing of knowledge seen between Alzheimer’s patients when they played a game together. All of these are positive interactions that Alzheimer’s patients typically don’t engage in.
Outlook
Maruyama discussed three key factors that will determine the outlook for Alzheimer’s drug research: how quickly new targets based on new approaches can be brought into the clinic, how willingly people will fund late-stage trials, and how well the right patients receive the right treatment (precision medicine). Patient selection for clinical trials will be particularly important if Alzheimer’s turns out to be a “cluster of related diseases” as certain treatments may be more effective in certain patients but not others.
“For me, the most exciting part of new targets being pursued is the diversification away from a more singular focus on amyloid,” Maruyama said. “I think it is likely that the next several years will see a dramatic redistribution of these approaches, with the addition of others that are not currently well enough understood or developed to be entering the clinic.”
“Even if tau or one of the other approaches shows a significant benefit, we are unlikely to find the silver bullet, a cure, in the form of a therapy that targets one protein,” Pfeifer commented. “It’s more likely that multiple points of intervention are going to be required to completely reverse and cure the disease.”
“It is often said that dementia drug discovery is now where cancer discovery was 15 years ago, as we come to the realization that we need to understand the basic biology better so that we can understand targets and approaches better,” Maruyama added. “If we can begin to understand patient segmentation in dementia the way we are beginning to understand it in oncology, we can make great progress.”
Maruyama explains that oncology had strong funding for basic cancer biology at the time, which allowed the field to move forward quickly with new treatments: “Dementia may be ready for a similar revolution, but we need to support more new scientists with new ideas to make that promise a reality.”
“Alzheimer’s disease is complicated, and we are still finding out many things about the condition, which we can only do through research,” Pfeifer said. “In fact, as in other areas of life, these drug failures are learning opportunities and we are learning more about the disease and how to treat it.”
While we continue to better understand Alzheimer’s and pursue effective drugs, having a healthy lifestyle and keeping active may help with disease onset and symptoms.
For more information about the disease and a current overview of the drug pipeline, check out the recently published BioSpace Alzheimer’s report.