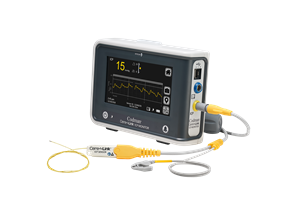
Integra LifeSciences Holdings Corporation is pleased to announce that following a successful Q1 2024 U.S. relaunch of its CereLink® ICP Monitoring System, the innovative product will be featured at the upcoming AANS Annual Scientific Meeting from May 3 through 6, 2024, in Chicago, Illinois.
Visit the Codman Specialty Surgical booth to learn how the CereLink ICP Monitoring System provides patients with uncompromised advanced continuous ICP monitoring.
PRINCETON, N.J., April 30, 2024 (GLOBE NEWSWIRE) -- Integra LifeSciences Holdings Corporation (Nasdaq: IART), a leading global medical technology company, is pleased to announce that following a successful Q1 2024 U.S. relaunch of its CereLink® ICP Monitoring System, the innovative product will be featured at the upcoming AANS Annual Scientific Meeting from May 3 through 6, 2024, in Chicago, Illinois.
The CereLink ICP Monitoring System provides clinicians with uncompromised advanced continuous ICP monitoring, with minimal drift, MR conditional capability, durable, flexible ICP sensors, and advanced data presentation features.1,2
“The relaunch of the CereLink ICP Monitoring System helps to address the important need for accurate monitoring of intracranial pressure, which is paramount when managing patients with traumatic brain injuries, intracranial hemorrhages, strokes and other neurological conditions,” said Mike McBreen, executive vice president and president, Codman Specialty Surgical. “This milestone represents a prime example of the dedication to our mission to innovate treatment pathways to advance patient outcomes and set new standards of care.”
As part of Integra’s industry-leading neurosurgical portfolio of products, the CereLink ICP Monitoring System will be highlighted in booth #655 at AANS 2024. To learn more, visit https://marketing.integralife.com/aans2024.
References: 1. CereLink bench test shows (2 stdev) of ±2.2 mmHg over 7 days and 3.8mmHg (4 stdev) over 7 days. 2. CereLink system IFUs
About Integra LifeSciences
At Integra LifeSciences, we are driven by our purpose of restoring patients’ lives. We innovate treatment pathways to advance patient outcomes and set new standards of surgical, neurologic and regenerative care. We offer a comprehensive portfolio of high quality, leadership brands that include Acclarent®, AmnioExcel®, Aurora®, Bactiseal®, BioD™, CerebroFlo®, CereLink® Certas® Plus, Codman®, CUSA®, Cytal®, DuraGen®, DuraSeal®, DuraSorb®, Gentrix®, ICP Express®, Integra®, Licox®, MAYFIELD®, MediHoney®, MicroFrance®, MicroMatrix®, NeuraGen®, NeuraWrap™, PriMatrix®, SurgiMend®, TCC-EZ® and VersaTru®. For the latest news and information about Integra and its products, please visit www.integralife.com.
Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 which involve risks and uncertainties and reflect the Company's judgment as of the date of this release. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements. These forward-looking statements are necessarily estimates reflecting the judgment of the Company’s management, including with respect to the U.S. relaunch of CereLink, and involve a number of risks and uncertainties that could cause actual results to differ materially from those suggested by the forward-looking statements. These forward-looking statements include, but are not limited to, statements related to the potential applications, improvements and efficacy of the Integra products described herein and the realization of improvements to patient standards of care and health outcomes. There can be no assurance these products will achieve the benefits described herein or that such benefits will be replicated. In addition, there can be no assurance that these products will be commercially successful. Forward-looking statements in this press release should be evaluated together with the many risks and uncertainties that affect Integra’s business and market which include but are not limited to: the Company’s ability to predict accurately the demand for products and products under development by it and to develop strategies to successfully address relevant markets; physicians’ willingness to adopt and third-party payers’ willingness to provide reimbursement for the Company’s products; fluctuations in hospitals' spending for capital equipment; difficulties or delays in manufacturing; the availability and pricing of third party sourced products and materials; global macroeconomic and political conditions, including acts of terrorism or outbreak of war, hostilities, civil unrest, and other political or security disturbances, including the State of Israel’s ongoing war against Hamas and any escalations of that conflict; and the economic, competitive, governmental, technological and other risk factors and uncertainties identified under the headings “Risk Factors” and “Special Note Regarding Forward-Looking Statements,” included in Integra's Annual Report on Form 10-K for the year ended December 31, 2023 and information contained in subsequent filings with the Securities and Exchange Commission. These forward-looking statements are made only as the date thereof, and Integra undertakes no obligation to update or revise the forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law.
Investor Relations:
Chris Ward
(609) 772-7736
chris.ward@integralife.com
Media Contact:
Laurene Isip
(609) 208-8121
laurene.isip@integralife.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d60afe37-3058-41db-aef2-84a921e1cf6c