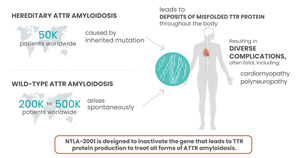
Intellia Therapeutics, Inc. and Regeneron Pharmaceuticals, Inc. announced positive interim data from an ongoing Phase 1 clinical study of their lead in vivo genome editing candidate, NTLA-2001, which is being developed as a single-dose treatment for transthyretin amyloidosis.
- First-ever clinical data supporting safety and efficacy of in vivo CRISPR genome editing in humans
- Interim readout in ongoing Phase 1 trial finds single 0.3 mg/kg dose of NTLA-2001 led to 87% mean reduction in serum TTR, with a maximum 96% serum TTR reduction by day 28, with dose-dependent response
- Encouraging safety profile; no serious adverse events observed in the first six patients by day 28
- Data published in The New England Journal of Medicine and presented at Peripheral Nerve Society Annual Meeting (PNS)
- Intellia to host investor event on Monday, June 28at 8:00 a.m. E.T.
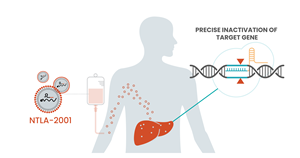
CAMBRIDGE, Mass. and TARRYTOWN, N.Y., June 26, 2021 (GLOBE NEWSWIRE) -- Intellia Therapeutics, Inc. (NASDAQ:NTLA) and Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) today announced positive interim data from an ongoing Phase 1 clinical study of their lead in vivo genome editing candidate, NTLA-2001, which is being developed as a single-dose treatment for transthyretin (ATTR) amyloidosis. The Phase 1 study, run by Intellia as the program’s development and commercialization lead, is evaluating NTLA-2001 in people living with hereditary transthyretin amyloidosis with polyneuropathy (ATTRv-PN). NTLA-2001 is the first CRISPR/Cas9-based therapy candidate to be administered systemically, via intravenous infusion, for precision editing of a gene in a target tissue in humans. NTLA-2001 is designed to inactivate the TTR gene in liver cells to prevent the production of misfolded transthyretin (TTR) protein, which accumulates in tissues throughout the body and causes the debilitating and often fatal complications of ATTR amyloidosis. The interim data were presented today at the 2021 Peripheral Nerve Society (PNS) Annual Meeting and published in The New England Journal of Medicine (nejm.org/doi/full/10.1056/NEJMoa2107454.)1.
“These are the first ever clinical data suggesting that we can precisely edit target cells within the body to treat genetic disease with a single intravenous infusion of CRISPR. The interim results support our belief that NTLA-2001 has the potential to halt and reverse the devastating complications of ATTR amyloidosis with a single dose,” said Intellia President and Chief Executive Officer John Leonard, M.D. “Solving the challenge of targeted delivery of CRISPR/Cas9 to the liver, as we have with NTLA-2001, also unlocks the door to treating a wide array of other genetic diseases with our modular platform, and we intend to move quickly to advance and expand our pipeline. With these data, we believe we are truly opening a new era of medicine.”
The interim data released today cover the first six ATTRv-PN patients across two single-ascending dose cohorts of the Phase 1 study, which is currently being conducted in the United Kingdom and New Zealand. Single doses of either 0.1 mg/kg or 0.3 mg/kg of NTLA-2001 were administered systemically. Reductions in serum TTR levels were measured from baseline to day 28. Treatment with NTLA-2001 led to dose-dependent reductions in serum TTR, with mean reductions of 52% among the three patients in the 0.1 mg/kg dose group, and 87% among the three patients in the 0.3 mg/kg dose group, including one patient with a 96% reduction. By contrast, the standard of care for ATTRv-PN, which requires chronic treatment, typically yields TTR reductions of approximately 80%.
“This is exciting early data both for people living with this devastating disease and for the entire scientific community working to maximize the potential of genetics-based medicines through cutting-edge research and technologies,” said George D. Yancopoulos, M.D., Ph.D., President and Chief Scientific Officer of Regeneron, which first partnered with Intellia in 2016 to advance CRISPR/Cas9 gene-editing technology for in vivo therapeutic development. “Thanks to large-scale human genetics research, there have been many new genetic targets identified and confirmed to have an impact on human health. Combining this knowledge with the precision and enhanced convenience of a single CRISPR infusion unlocks new possibilities in treating – and potentially even curing – life-threatening and historically difficult-to-address diseases.”
At both dose levels, NTLA-2001 was generally well-tolerated by the six patients included in the interim analysis, with no serious adverse events and no liver findings by day 28. Given the safety and tolerability profile so far, NTLA-2001 is continuing to be evaluated in the dose-escalation portion of the study, to determine if a higher dose could result in a deeper reduction in disease-causing protein levels leading to the potential for more meaningful clinical benefit. As of the date of this release, Cohort 3, evaluating NTLA-2001 at the 1 mg/kg dose level, is actively enrolling.
Following the identification of a recommended dose in the dose-escalation portion of the study, Intellia expects to begin a single-dose expansion cohort in Part 2 of the Phase 1 trial later this year. After completion of the Phase 1 trial, the company plans to move to pivotal studies for both polyneuropathy and cardiomyopathy manifestations of ATTR amyloidosis.
“ATTR amyloidosis is a progressive and fatal disease that usually requires chronic, lifelong treatment. These interim Phase 1 data support NTLA-2001 as the only one-time treatment either on the market or in development,” said Julian Gillmore, M.D., Ph.D., Professor of Medicine, National Amyloidosis Centre, UCL Division of Medicine, Royal Free Hospital, U.K., and the Phase 1 study’s national coordinating investigator. “As the first-ever systemically administered CRISPR therapy candidate, NTLA-2001 shows strong potential to stop the production and accumulation of the misfolded TTR protein by inactivating the TTR gene at the root of the disease. This approach could deliver life-changing, lifelong benefits to patients with all forms of ATTR amyloidosis, who continue to experience debilitating symptoms and complications of disease while on the standard of care. While further investigation is needed, these results are highly encouraging.”
Intellia intends to present additional data from the study at a medical or scientific meeting later this year.
1 Gillmore JD, Gane E, Taubel J, et al. CRISPR /Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. NEngl J Med 2021. nejm.org/doi/full/10.1056/NEJMoa2107454
Intellia Therapeutics Investor Event and Webcast Information
Intellia will host a live webcast on Monday, June 28, 2021 at 8:00 a.m. E.T. to review the presented data. To join the webcast, please visit this link, or the Events and Presentations page of the Investors & Media section of the company’s website at www.intelliatx.com. A replay of the webcast will be available on Intellia’s website for at least 30 days following the call.
About NTLA-2001
Based on Nobel Prize-winning CRISPR/Cas9 technology, NTLA-2001 could potentially be the first curative treatment for ATTR amyloidosis. NTLA-2001 is the first investigational CRISPR therapy candidate to be administered systemically, or through a vein, to edit genes inside the human body. Intellia’s proprietary non-viral platform deploys lipid nanoparticles to deliver to the liver a two-part genome editing system: guide RNA specific to the disease-causing gene and messenger RNA that encodes the Cas9 enzyme, which carries out the precision editing. Robust preclinical data, showing deep and long-lasting transthyretin (TTR) reduction following in vivo inactivation of the target gene, supports NTLA-2001’s potential as a single-administration therapeutic. Interim Phase 1 clinical data released in June 2021 confirm substantial, dose-dependent reduction of TTR protein following a single dose of NTLA-2001. Intellia leads development and commercialization of NTLA-2001 as part of a multi-target discovery, development and commercialization collaboration with Regeneron.
About the NTLA-2001 Clinical Program
The global Phase 1 trial is an open-label, multi-center, two-part study of NTLA-2001 in adults with hereditary transthyretin amyloidosis with polyneuropathy (ATTRv-PN). The trial’s primary objectives are to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of NTLA-2001. Patients receive a single dose of NTLA-2001 via intravenous administration. The study will enroll up to 38 participants (ages 18-80 years) and consists of a single-ascending dose phase followed by a dose-expansion phase, which is expected to begin later in 2021. Visit clinicaltrials.gov (NCT04601051) or eudract.ema.europa.eu/ (2020-002034-32) for more details.
Enrollment is ongoing at clinical trial sites in the U.K. and New Zealand, and Intellia is submitting additional regulatory applications to support a planned global study expansion. After completion of the Phase 1 trial, the company plans to move to pivotal studies for both polyneuropathy and cardiomyopathy manifestations of ATTR amyloidosis.
About Transthyretin (ATTR) Amyloidosis
Transthyretin amyloidosis, or ATTR amyloidosis, is a rare, progressive and fatal disease. Hereditary ATTR (ATTRv) amyloidosis occurs when a person is born with mutations in the TTR gene, which causes the liver to produce structurally abnormal transthyretin (TTR) protein with a propensity to misfold. These damaged proteins build up as amyloid in the body, causing serious complications in multiple tissues, including the heart, nerves and digestive system. ATTRv amyloidosis predominantly manifests as polyneuropathy (ATTRv-PN), which can lead to nerve damage, or cardiomyopathy (ATTRv-CM), which can lead to heart failure. Some individuals without the genetic mutation produce non-mutated, or wild-type TTR proteins that become unstable over time, misfolding and aggregating in disease-causing amyloid deposits. This condition, called wild-type ATTR (ATTRwt) amyloidosis, primarily affects the heart. There are an estimated 50,000 people worldwide living with ATTRv amyloidosis and between 200,000 and 500,000 people with ATTRwt amyloidosis.
About Intellia Therapeutics
Intellia Therapeutics, a leading clinical-stage genome editing company, is developing novel, potentially curative therapeutics using CRISPR/Cas9 technology. To fully realize the transformative potential of CRISPR/Cas9, Intellia is pursuing two primary approaches. The company’s in vivo programs use intravenously administered CRISPR as the therapy, in which proprietary delivery technology enables highly precise editing of disease-causing genes directly within specific target tissues. Intellia’s ex vivo programs use CRISPR to create the therapy by removing, re-engineering and re-infusing the patient’s own cells to treat cancer and autoimmune diseases. Intellia’s deep scientific, technical and clinical development experience, along with its robust intellectual property portfolio, have enabled the company to take a leadership role in harnessing the full potential of CRISPR/Cas9 to create new classes of genetic medicine. Learn more at intelliatx.com. Follow us on Twitter @intelliatweets.
About Regeneron
Regeneron (NASDAQ: REGN) is a leading biotechnology company that invents life-transforming medicines for people with serious diseases. Founded and led for over 30 years by physician-scientists, our unique ability to repeatedly and consistently translate science into medicine has led to nine FDA-approved treatments and numerous product candidates in development, almost all of which were homegrown in our laboratories. Our medicines and pipeline are designed to help patients with eye diseases, allergic and inflammatory diseases, cancer, cardiovascular and metabolic diseases, pain, hematologic conditions, infectious diseases and rare diseases.
Regeneron is accelerating and improving the traditional drug development process through our proprietary VelociSuite® technologies, such as VelocImmune®, which uses unique genetically humanized mice to produce optimized fully human antibodies and bispecific antibodies, and through ambitious research initiatives such as the Regeneron Genetics Center, which is conducting one of the largest genetics sequencing efforts in the world.
For additional information about the company, please visit www.regeneron.com or follow @Regeneron on Twitter.
Intellia Forward-Looking Statements
This press release contains “forward-looking statements” of Intellia Therapeutics, Inc. (“Intellia”, “we” or “our”) within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, express or implied statements regarding Intellia’s beliefs and expectations regarding its: ability to enroll and dose the necessary subjects in the clinical studies for NTLA-2001 for the treatment of transthyretin amyloidosis (“ATTR”), provide timing on data readouts from the clinical studies, and successfully secure additional clinical studies authorizations, such as investigational new drug applications (“IND”) and clinical trial applications (“CTA”), in other countries; ability to evaluate NTLA-2001 in a broader ATTR population; expectation that clinical results will support NTLA-2001’s safety and activity profile; belief that NTLA-2001 can be approved as a single-dose therapy or that it can halt and reverse ATTR progression; plans to present data at upcoming scientific conferences; advancement, expansion and acceleration of our CRISPR/Cas9 technology and in vivo pipeline to develop breakthrough genome editing treatments for people living with severe diseases; ability to demonstrate our platform’s modularity and replicate or apply results achieved in preclinical studies, including those in our ATTR program, in any future studies, including human clinical trials; ability to optimize the impact of our collaborations on our development programs, including but not limited to our collaboration with Regeneron Pharmaceuticals, Inc. (“Regeneron”); statements regarding the timing of regulatory filings and clinical trial execution, including dosing of patients, regarding our development programs; and potential commercial opportunities, including value and market, for our product candidates.
Any forward-looking statements in this press release are based on management’s current expectations and beliefs of future events, and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: risks related to our ability to protect and maintain our intellectual property position; risks related to our relationship with third parties, including our licensors and licensees; risks related to the ability of our licensors to protect and maintain their intellectual property position; uncertainties related to regulatory agencies’ evaluation of regulatory filings and other information related to our product candidates; uncertainties related to the authorization, initiation and conduct of studies and other development requirements for our product candidates; the risk that any one or more of our product candidates, including those that are co-developed, will not be successfully developed and commercialized; the risk that the results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies; and the risk that our collaborations with Regeneron or our other ex vivo collaborations will not continue or will not be successful. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause Intellia’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Intellia’s most recent annual report on Form 10-K and quarterly report on Form 10-Q, as well as discussions of potential risks, uncertainties, and other important factors in Intellia’s other filings with the Securities and Exchange Commission (“SEC”). All information in this press release is as of the date of the release, and Intellia undertakes no duty to update this information unless required by law.
Regeneron Forward Looking Statements
This press release includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the impact of SARS-CoV-2 (the virus that has caused the COVID-19 pandemic) on Regeneron’s business and its employees, collaborators, and suppliers and other third parties on which Regeneron relies, Regeneron’s and its collaborators’ ability to continue to conduct research and clinical programs, Regeneron’s ability to manage its supply chain, net product sales of products marketed or otherwise commercialized by Regeneron and/or its collaborators (collectively, “Regeneron’s Products”), and the global economy; the nature, timing, and possible success and therapeutic applications of Regeneron’s Products and product candidates being developed by Regeneron and/or its collaborators (collectively, “Regeneron’s Product Candidates”) and research and clinical programs now underway or planned, such as NTLA-2001 (a product candidate being developed for transthyretin (ATTR) amyloidosis under a multi-target discovery, development, and commercialization collaboration between Regeneron and Intellia Therapeutics, Inc.); the extent to which the results from the research and development programs conducted by Regeneron and/or its collaborators (including the Phase 1 clinical study evaluating NTLA-2001 discussed in this press release) may be replicated in other studies and/or lead to advancement of product candidates to clinical trials, therapeutic applications, or regulatory approval; the potential of the CRISPR/Cas9 gene-editing technology discussed in this press release for in vivo therapeutic development; uncertainty of the utilization, market acceptance, and commercial success of Regeneron’s Products and Regeneron’s Product Candidates and the impact of studies (whether conducted by Regeneron or others and whether mandated or voluntary), including the Phase 1 clinical study evaluating NTLA-2001 discussed in this press release, on any of the foregoing or any potential regulatory approval of Regeneron’s Products and Regeneron’s Product Candidates (such as NTLA-2001); the likelihood, timing, and scope of possible regulatory approval and commercial launch of Regeneron’s Product Candidates and new indications for Regeneron’s Products; the ability of Regeneron’s collaborators, suppliers, or other third parties (as applicable) to perform manufacturing, filling, finishing, packaging, labeling, distribution, and other steps related to Regeneron’s Products and Regeneron’s Product Candidates; the ability of Regeneron and/or its collaborators to manufacture and manage supply chains for multiple products and product candidates; safety issues resulting from the administration of Regeneron’s Products and Regeneron’s Product Candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s Products and Regeneron’s Product Candidates (such as NTLA-2001) in clinical trials; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s Products and Regeneron’s Product Candidates; ongoing regulatory obligations and oversight impacting Regeneron’s Products, research and clinical programs, and business, including those relating to patient privacy; the availability and extent of reimbursement of Regeneron’s Products from third-party payers, including private payer healthcare and insurance programs, health maintenance organizations, pharmacy benefit management companies, and government programs such as Medicare and Medicaid; coverage and reimbursement determinations by such payers and new policies and procedures adopted by such payers; competing drugs and product candidates that may be superior to, or more cost effective than, Regeneron’s Products and Regeneron’s Product Candidates; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its financial projections or guidance and changes to the assumptions underlying those projections or guidance; the potential for any license, collaboration, or supply agreement, including Regeneron’s agreements with Sanofi, Bayer, and Teva Pharmaceutical Industries Ltd. (or their respective affiliated companies, as applicable), as well as Regeneron’s collaboration with Intellia Therapeutics, Inc. discussed in this press release, to be cancelled or terminated; and risks associated with intellectual property of other parties and pending or future litigation relating thereto (including without limitation the patent litigation and other related proceedings relating to EYLEA® (aflibercept) Injection, Dupixent® (dupilumab), Praluent® (alirocumab), and REGEN-COVTM (casirivimab and imdevimab)), other litigation and other proceedings and government investigations relating to the Company and/or its operations, the ultimate outcome of any such proceedings and investigations, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the year ended December 31, 2020 and its Form 10-Q for the quarterly period ended March 31, 2021. Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update (publicly or otherwise) any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise.
Regeneron uses its media and investor relations website and social media outlets to publish important information about the Company, including information that may be deemed material to investors. Financial and other information about Regeneron is routinely posted and is accessible on Regeneron’s media and investor relations website (http://newsroom.regeneron.com) and its Twitter feed (http://twitter.com/regeneron).
Intellia Contacts
Media:
Matt Crenson
Ten Bridge Communications
+1-917-640-7930
media@intelliatx.com
mcrenson@tenbridgecommunications.com
Investors:
Lina Li
Director, Investor Relations
+1-857-706-1612
lina.li@intelliatx.com
Regeneron Contacts
Media:
Alexandra Bowie
+1-202-213-1643
alexandra.bowie@regeneron.com
Investors:
Vesna Tosic
+1-914-847-5443
Vesna.tosic@regeneron.com
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/63fedc33-fa6e-4e69-86ed-1af39f985ce4
https://www.globenewswire.com/NewsRoom/AttachmentNg/fc4b69d0-143d-4cf1-a9a2-0813fa9097b0
https://www.globenewswire.com/NewsRoom/AttachmentNg/32d6508d-69a7-4cea-8bed-aac3a6df2e7f
https://www.globenewswire.com/NewsRoom/AttachmentNg/f716182f-320b-44b1-a8e8-1ace4097493e
https://www.globenewswire.com/NewsRoom/AttachmentNg/cb29f5f1-2f1e-4e7d-ad70-8b9324295ff4