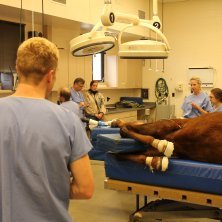
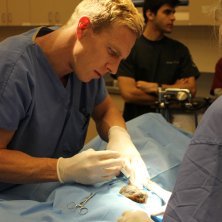
RICHLAND, WA--(Marketwired - December 08, 2014) - IsoRay Inc. (NYSE MKT: ISR), a medical technology company and innovator in brachytherapy and medical isotope applications, today announced the world's first veterinary implant of a horse suffering from cancer utilizing IsoRay's Cesium-131 internal radiation (brachytherapy) cancer therapy.
"Forty Five Caliber" (better known as "LB") is an 8 year old American Saddlebred gelding, who formerly competed throughout the U.S. as a show horse. Unfortunately, in 2012, he developed an abnormal cancerous growth on his left upper eyelid. After this growth continued to enlarge, he was evaluated by an equine ophthalmologist who performed a surgical resection of the tumor, which turned out to be a malignant peripheral nerve sheath tumor. Within four months, the tumor had re-grown to its original size. Despite three subsequent attempts at surgical resection, and a chemotherapy regimen of five injections, LB's tumor progression continued. Dr. Jonathan Feddock at the University Of Kentucky Department Of Radiation Oncology was contacted to evaluate the horse for the possibility of radiation therapy.
In October 2014, Dr. Feddock examined "LB" and noted the tumor covered the entire upper eyelid in a teardrop shape extending laterally along the bony rim. The horse was no longer able to close his eyelid and required the owner to administer saline drops daily to limit dry eye and further problems.
"There currently are no veterinary radiation oncology services in the State of Kentucky, but the equine hospital where the horse was being treated, Rood and Riddle Equine Hospital has nuclear imaging capabilities, so I realized there would be an opportunity to possibly perform a permanent implant," says Dr. Feddock.
"The tumor was freely mobile, and easy to palpate and maneuver, so I figured Cesium-131 seeds would be the best and most appropriate treatment given the circumstances."
At Rood and Riddle Equine Hospital the horse was put under general anesthesia, and Dr. Feddock, with the assistance of the veterinary ophthalmologist, performed a permanent interstitial implant using Cesium-131 into the horse's upper eyelid. "I calculated the seed strength and number to deliver a dose of 65 Gy to a depth of 5mm, which is a curative dose of radiation for this type of tumor."
Dr. Feddock was initially approached about performing this type of an implant because of his previous success with placing similar permanent interstitial implants with Cesium-131 in human gynecologic cancers. "The basic physics and clinical concepts of dosing and placing the radiation sources were the same as for humans, but the horse's skin was definitely much tougher than I am used to."
"The reason I chose Cesium-131 for this particular equine application was because of multiple factors. As the horse required anesthesia, I obviously could not transport the horse to my treatment vault to perform external beam treatments, and the horse owner was unable to drive several states away to the nearest equine radiation oncology clinic. With Cesium-131 seeds, you can perform the required surgery and place the seeds in a single procedure. LB holds a tremendous amount of sentimental value to the family, which includes children, so radiation exposure was very important and Cesium-131 has a very short half-life of 9.7 days. A permanent implant using Cesium-131 seemed to address all of these factors - single procedure, low morbidity, curative treatment, and a very low exposure for not only all of the personnel involved but also to the family for the upcoming holidays," says Feddock. "Considering this is a tumor with a relatively slow cell cycle, it is my hope that in the next few months we will see the tumor regress considerably." LB has already had his first follow-up visit, and one week after treatment, he has had minimal side effects."
The owner of the horse was very thankful for the procedure and commented, "I am very happy that Dr. Feddock has been able to provide his expertise and services, including offering newer treatments that are otherwise not frequently performed in horses."
IsoRay Chairman and CEO Dwight Babcock commented, "Having ranched for over 30 years I hold a deep awareness of the trust and care bestowed upon us to care for our animals. There is a unique unconditional bond between humans and animals that drives our need to care for them. I can only hope that our unique isotope Cesium-131 can give a chance at a better quality of life for those animals afflicted with cancer."
For humans, IsoRay's various products, including Cesium-131 seeds, sutured seeds, stranded mesh and the GliaSite® radiation therapy system, give physicians the ability to directly place a specified dosage of radiation in areas where cancer is most likely to remain after completion of a tumor removal, directly into the tumor or by placing seeds within the prostate or tumor. The ability to precisely place a specified dose of radiation means there is less likelihood for damage to occur to healthy surrounding tissue compared to other alternative treatments. IsoRay's cancer fighting products diminish the ability of the tumor to recur, resulting in important benefits for patients in longevity as well as quality of life.
IsoRay is the exclusive manufacturer of Cesium-131. The pioneering brachytherapy is one of the most significant advances in internal radiation therapy in 20 years. Cesium-131 allows for the precise treatment of many different cancers because of its unrivaled blend of high energy and its 9.7 day half-life (its unequaled speed in giving off therapeutic radiation).
In addition to its CMS codes, Cesium-131 is FDA-cleared and holds a CE mark for international sales in seed form for the treatment of brain cancer, prostate cancer, lung cancer, ocular melanoma cancer, colorectal cancer, gynecologic cancer, head and neck cancer and other cancers throughout the body. The treatment can be deployed using several delivery methods including single seed applicators, implantable strands and seed sutured mesh. IsoRay also sells several new implantable devices, including the GliaSite® radiation therapy system.
About IsoRay
IsoRay, Inc., through its subsidiary, IsoRay Medical, Inc. is the sole producer of Cesium-131 brachytherapy seeds, which are expanding brachytherapy options throughout the body. Learn more about this innovative Richland, Washington company and explore the many benefits and uses of GliaSite® and Cesium-131 by visiting www.isoray.com. Join us on Facebook/Isoray. Follow us on Twitter @Isoray.
Safe Harbor Statement
Statements in this news release about IsoRay's future expectations, including: the advantages of our products and their delivery systems, whether IsoRay will be able to continue to expand its base beyond prostate cancer, whether the use of our products will increase or continue, whether awareness of our products in the veterinary community will continue or increase, whether future veterinary treatment of various cancers using our products will have favorable results, and all other statements in this release, other than historical facts, are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 ("PSLRA"). This statement is included for the express purpose of availing IsoRay, Inc. of the protections of the safe harbor provisions of the PSLRA. It is important to note that actual results and ultimate corporate actions could differ materially from those in such forward-looking statements based on such factors as physician acceptance, training and use of our products, our ability to successfully manufacture, market and sell our products, our ability to manufacture our products in sufficient quantities to meet demand within required delivery time periods while meeting our quality control standards, our ability to enforce our intellectual property rights, whether additional studies are released and support the conclusions of past studies, whether ongoing patient results (both animal and human) with our products are favorable and in line with the conclusions of clinical studies and initial patient results, patient results achieved when our products are used for the treatment of cancers and malignant diseases beyond prostate, successful completion of future research and development activities, whether we, our distributors and our customers will successfully obtain and maintain all required regulatory approvals and licenses to market, sell and use our products in its various forms, continued compliance with ISO standards as audited by BSI, the success of our sales and marketing efforts, changes in reimbursement rates, changes in laws and regulations applicable to our products, and other risks detailed from time to time in IsoRay's reports filed with the SEC.
Help employers find you! Check out all the jobs and post your resume.