Jubilant Therapeutics’ differentiated, orally bioavailable molecules address both validated and novel therapeutic targets in oncology and immunology.
Chronic myeloid leukemia, red blood cell blood smear/iStock
With two Orphan Drug Designations, an advantageous safety profile and enhanced therapeutic indices, Jubilant Therapeutics is gearing up for Phase II and first-in-human trials later in 2023.
Jubilant’s pipeline is generated 100% in-house using their Therapeutic Index and Brain Exposure Optimization (TIBEO) discovery engine, which permits the identification of candidate molecules with enhanced therapeutic indices and, where beneficial, the ability to penetrate the brain to achieve pharmacologically active concentrations. TIBEO brings together a number of aspects such as structure-based drug design, computational modeling, genetic signatures and the expertise of Jubilant Therapeutics (JTI)'s research team, which has decades of successful drug discovery experience.
This approach makes drug discovery JTI’s core strength. Therefore, their strategic objective is to develop candidates through clinical proof of concept and then partner those assets with companies that have the expertise to advance them through late-stage clinical development and to market.
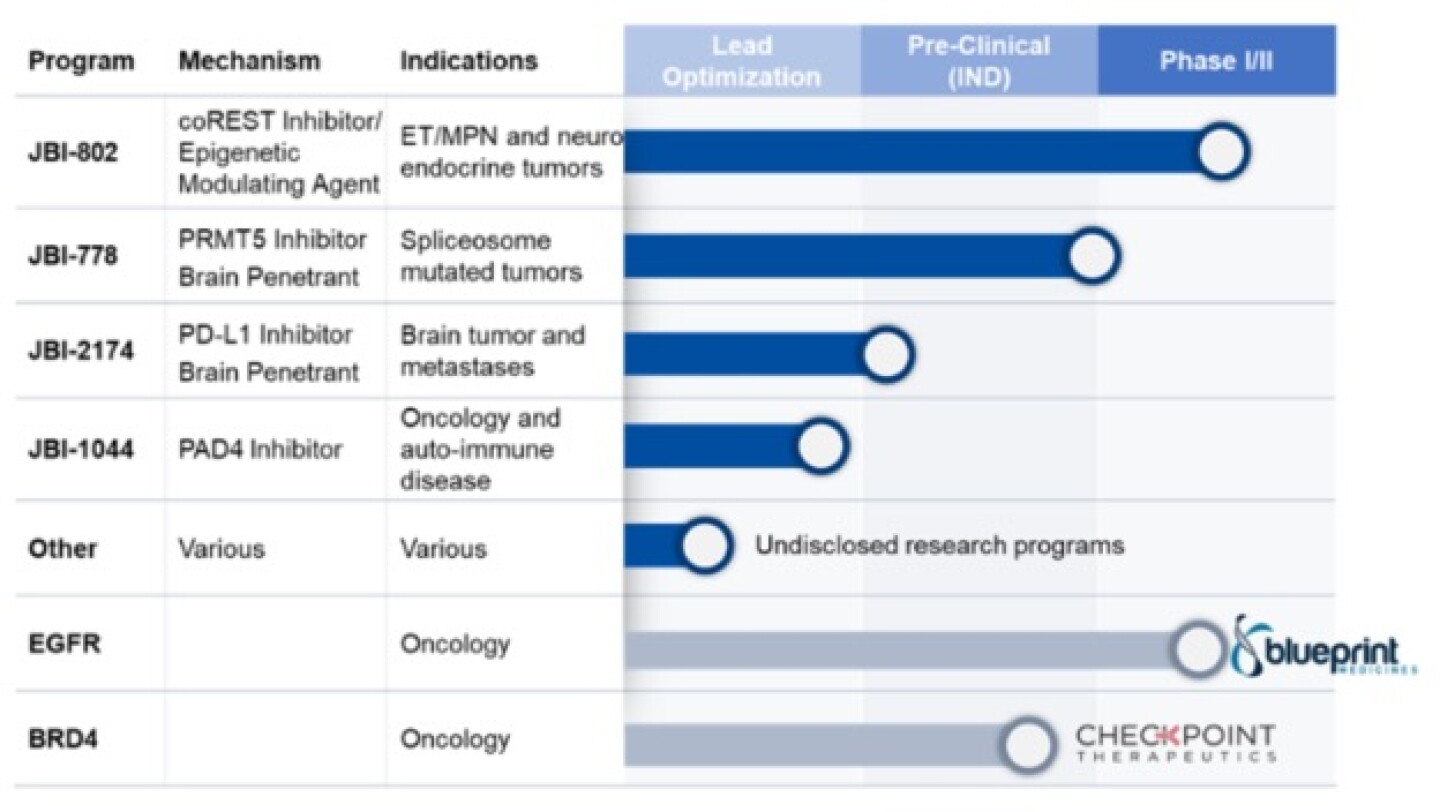
Figure 1 - Broad pipeline of differentiated assets with improved therapeutic indexes
President and Chief Executive Officer Syed Kazmi, a biopharmaceutical executive with more than 30 years in industry leadership roles at J&J, Ligand, Amgen and Novartis, has led Jubilant since its spinoff from a large global healthcare organization in 2019 and establishment in New Jersey as a precision oncology and immunology focused biotech. At Jubilant, he has brought together a team of exceptional scientists that have developed from scratch a strong pipeline anchored by their four most advanced clinical and preclinical programs (Figure 1), a unique mix of differentiated molecules addressing validated but difficult-to-drug targets as well as first-in-class targets.
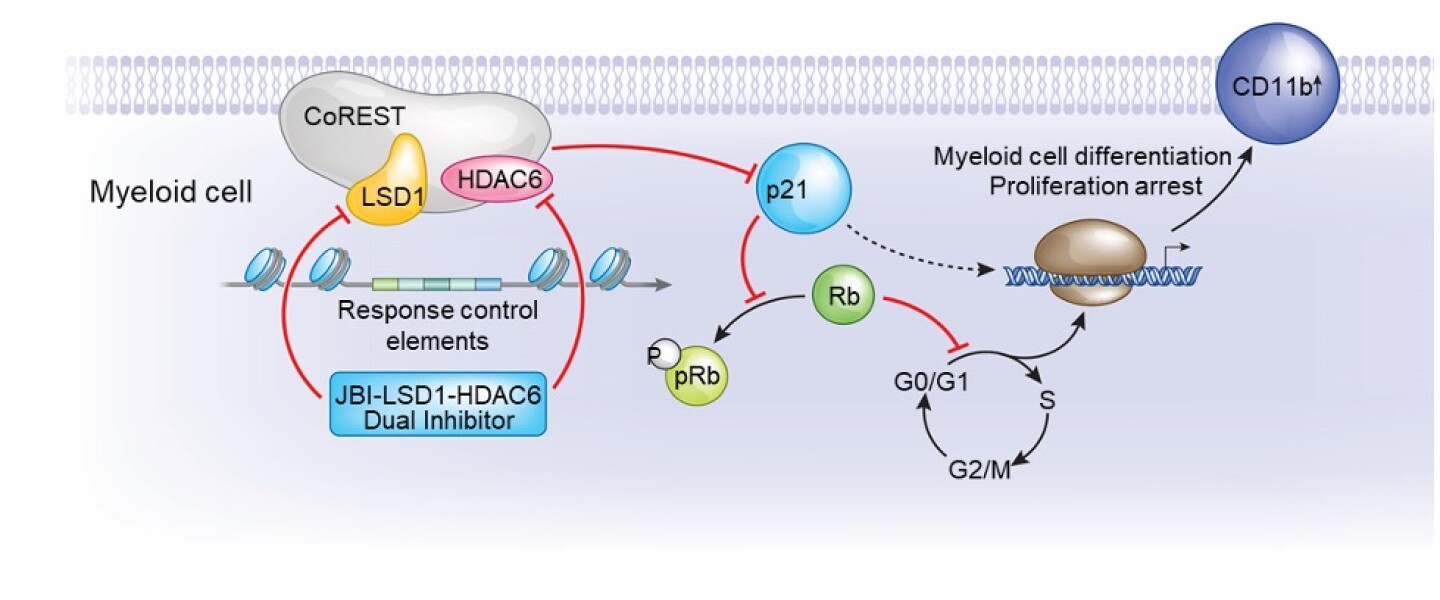
Figure 2 - JBI-802 is a novel and potent inhibitor of coREST in which dual inhibition of LSD1 and HDAC6 enables cell proliferation arrest by inducing p21 and myeloid progenitor cell differentiation, as shown by CD11b induction.
The lead program, JBI-802, is an oral inhibitor of coREST, an emerging target that is implicated in the development of hematological tumors like essential thrombocythemia (ET), certain neuroendocrine tumors and in the resistance to immune-checkpoint inhibition.
This unique compound was created by achieving the optimal, concomitant and selective inhibition of 2 components of coREST, lysine specific demethylase 1 (LSD1) and histone deacetylase 6 (HDAC6) (Figure 2). Malfunction of the coREST complex affects the maturation of cells in the platelet and erythroid lineages. It also induces an undifferentiated, proliferative phenotype typical of myeloproliferative neoplasms (MPN) and related subtypes of acute myeloid leukemia (AML). Inhibition of coREST induces differentiation and death of these MPNs and leukemia progenitors, giving it the potential to achieve an anti-tumor activity in these diseases. The U.S. Food and Drug Administration has granted Orphan Drug Designation for JBI-802 for the treatment of neuroendocrine small cell lung cancer (SCLC) and AML.
In preclinical testing, JBI-802’s targeting of coREST in animal models generated anti-tumor activity superior to that achieved by inhibitors of either LSD1 or HDAC6 alone. JBI-802 showed a favorable safety profile especially, with no effect on red blood cells and therefore no anemia. This compound is presently in Phase I/II clinical trials for the treatment of neuroendocrine, solid tumors that are known to depend on coREST, and the preliminary clinical data appears to confirm this lack of anemia in humans.
This preliminary data has also shown a dose-dependent decrease in platelets which constitute a proof-of-principle for the development of JBI-802 in essential thrombocythemia.
It is therefore important to compare JBI-802 with the most advanced LSD1–only inhibitor, bomedemstat, developed for the treatment of ET by Imago Biosciences, recently acquired by Merck for $1.35B.
JBI-802, by targeting coREST via both LSD1 and HDAC6, has a wider mechanism of action, affecting megakaryocytic, erythroid lineages and related tumors. The dual targeting has a superior, synergistic antitumor activity nicely demonstrated in animal models of erythroleukemia.
In addition to not showing the anemia observed with bomedemstat, JBI-802’s emerging safety profile also does not appear to show any dysgeusia (loss of taste), the most common toxicity reported by bomedemstat.
Building on the preclinical and clinical results, a follow-up Phase II trial is planned to start in the second half of 2023 to evaluate JBI-802 in ET, other MPNs and those tumors that have the mixed phenotype of MPN and myelodysplastic syndromes (MDS/MPN syndrome) where lack of induced anemia could represent a major benefit compared with standard of care.
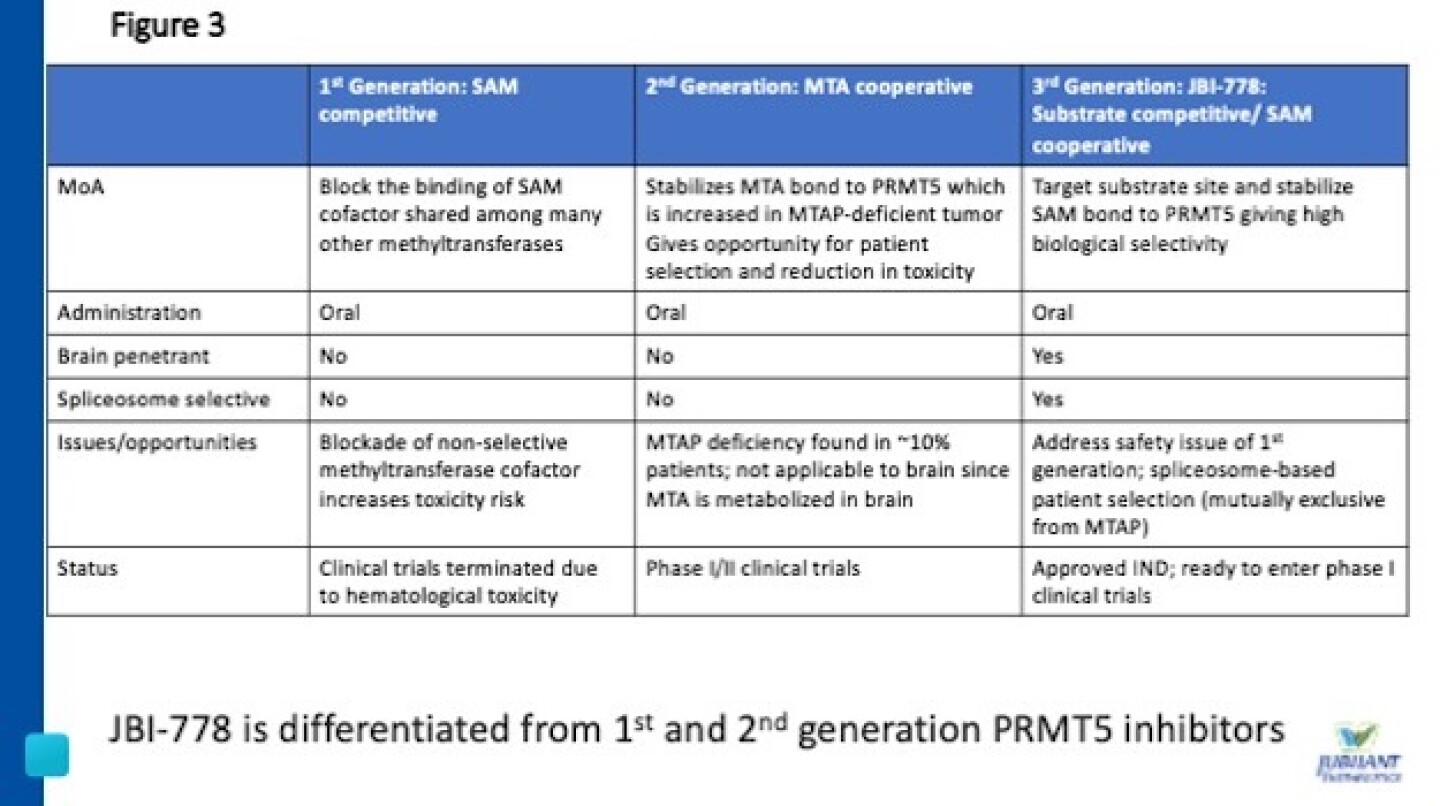
Figure 3 - JBI-778 is differentiated from 1st and 2nd generation PRMT5 inhibitors
Jubilant’s second clinical-stage drug candidate is JBI-778, a third-generation protein arginine methyltransferase 5 (PRMT5) inhibitor that is highly differentiated from first and second-generation PRMT5 inhibitors (Figure 3).
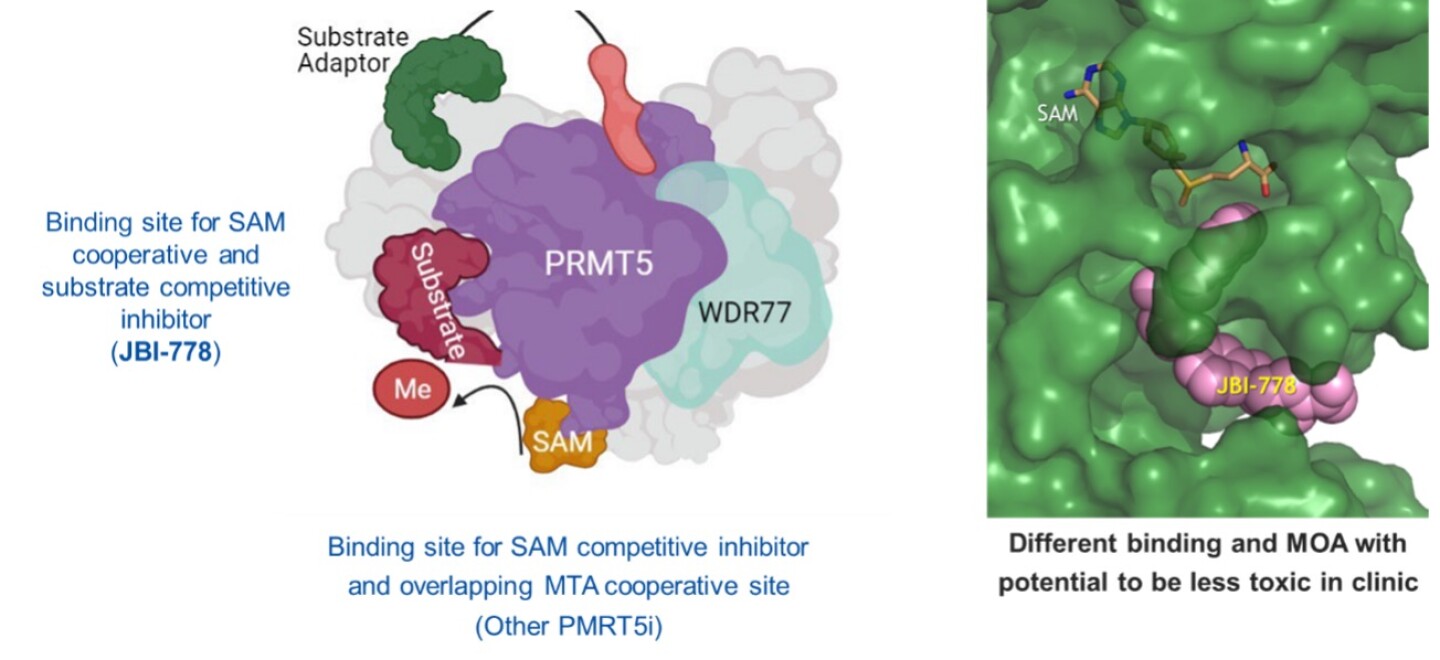
Figure 4- JBI-778 is an oral, highly differentiated, substrate competitive PRMT5 inhibitor
JBI-778 is substrate competitive, SAM cooperative, spliceosome selective and brain penetrant. JBI-778 targets the substrate site and stabilizes SAM bound to PRMT5, providing high biological selectivity (Figure 4) and has shown a superior safety profile as seen in animals.
By targeting the substrate site, JBI-778 is optimally designed to target tumors with mutations in the spliceosome proteins, a high unmet medical need with limited or no approved therapies. Spliceosome protein mutations are common in a subset of MDS, AML and are present in about 10% of lung tumors.
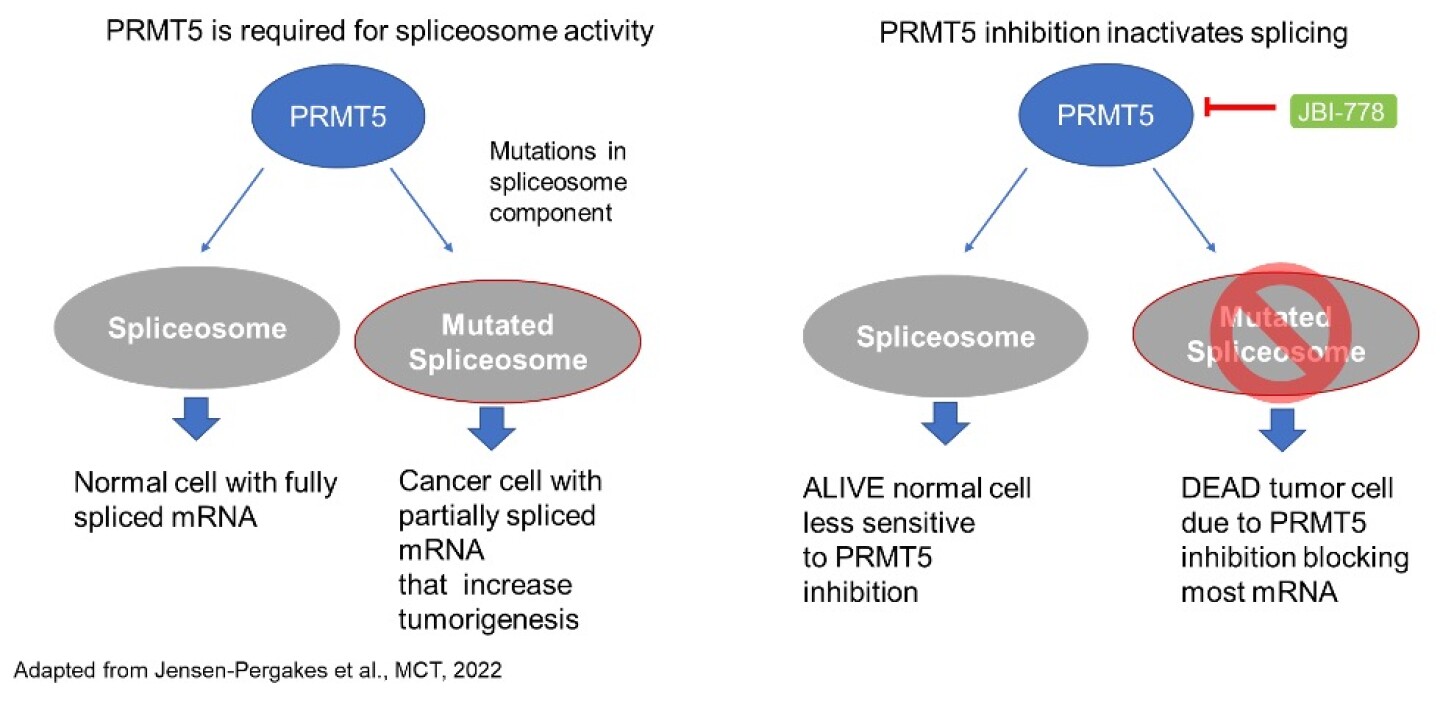
Spliceosome proteins are among the major substrates of PRMT5, which is required to activate their ability to produce fully spliced, normal RNAs. Indeed, it has been possible to show synthetic lethality between PRMT5 inhibition and spliceosome protein mutations (Figure 5). Tumor cells with spliceosome mutations are killed by optimal levels of JBI-778 inhibition due to accumulation of misplaced RNA. However, normal cells remain unaffected at the same level of JBI-778.
Figure 5 - Spliceosome mutations create synthetic lethality with PRMT5 inhibition
JBI-778 also demonstrates high brain exposure in animal models, which provides the opportunity to treat the increasing number of patients with lung tumors that have both spliceosome mutations and brain metastasis.
JBI-778 has an open investigational new drug application and a first-in-human clinical study focusing on tumors with spliceosome mutations in both hematological tumors and solid tumors such as lung cancer is expected to be initiated later this year. The FDA has also granted JBI-778 Orphan Drug Designation for the treatment of GBM (glioblastoma).
Jubilant has two other disclosed programs in preclinical development on IND track. One is a first-in-class peptidylarginine deiminase 4 (PAD4) inhibitor in development for autoimmune and oncology indications. PAD4 catalyzes the conversion of arginine to citrulline, and there is strong evidence that citrullinated proteins play a causal role in inflammatory and autoimmune diseases, including the establishment of liver and lung metastasis.
Jubilant, internally and in collaboration with external collaborators such as Boston Children’s Hospital, has shown that PAD4 inhibitors are very active in multiple animal models of rheumatoid arthritis, lung fibrosis, psoriasis and diabetic wound healing.
Inhibition of PAD4 is a potential breakthrough for these inflammatory and autoimmune diseases because, unlike current standard of care, inhibition of PAD4 does not result in immunosuppression.
Another preclinical stage program is JBI-2174, a first-in-class, oral, brain penetrant PD-L1 inhibitor under development for the treatment of tumors localized in the brain, including primary brain tumors, brain and leptomeningeal metastases.
In vivo, JBI-2174 shows similar high concentrations in plasma and the brain. Existing PD-L1 (and PD1) inhibitor antibodies are unable to pass through the blood-brain barrier to enter the brain and thus have limited efficacy in these tumors. With its optimized brain penetration and accumulation, JBI-2174 could represent a breakthrough for immunotherapy for CNS tumors. JBI-2174 has shown strong, immune-based antitumor activity in systemic and brain-localized animal models. An IND submission is planned for 2023.
Guided by world-renowned scientific advisors and a stellar board, Jubilant’s strategy includes partnering novel pipeline programs at appropriate inflection points to bring the drugs faster to market globally and to patients most in need. To date, Jubilant has outlicensed a novel EGFR Exon 20 pre-clinical program to Lengo Therapeutics, launched by Frazier Healthcare Partners. Lengo was recently acquired by Blueprint Medicines for $250 million upfront and $215 million in future milestones. A second program, bromodomain 4 (BRD4) inhibition, is partnered with Checkpoint Therapeutics.
If you wish to learn more about Jubilant’s pipeline and programs, please contact the company at BD@jubilanttx.com or visit www.jubilanttx.com.