With new features that make it faster and easier to use, Novoxel Inc. announces the launch of Tixel 2® to the USA.
KNOXVILLE, Tenn., June 5, 2024 /PRNewswire/ -- With new features that make it faster and easier to use, Novoxel Inc. announces the launch of Tixel 2® to the USA.
- Unlike devices that use laser or light energy, Tixel® systems use proprietary Thermo-Mechanical Action (TMA®). Therefore, Tixel can be used all year round – even during summer.
- Furthermore, Tixel is US FDA 510(k) cleared for fractional resurfacing of all skin types.
- Doctors use Tixel for a wide variety of difficult conditions – from tough "lip line" wrinkles to delicate periorbital skin.
SUNNY REGIONS & DARKER SKIN: Tixel is popular in Asia, Latin America, the Middle East, etc. When the previous version was introduced to the USA, word of its effectiveness spread rapidly across the "sunshine states" (Florida, Texas, California, etc.) and over 500 systems were sold within 2 years. Lee Pannell, CEO of Novoxel Inc., explained, "Where traditional devices have restrictions in sunny regions or darker skin types, Tixel has filled their void. With Tixel's wide range of applications, thousands of clinicians worldwide are delivering the benefits of our innovative technology."
VERSATILE & REPRODUCIBLE: Novoxel® strives to continually enhance fractional treatments and has invested in a myriad of clinical studies. As one outcome, Tixel received US FDA 510(k) clearance for periorbital wrinkles. In addition, nearly 30 peer-reviewed publications report the reproducibility of Tixel across a wide variety of conditions. Steven Weiner MD, a plastic surgeon in Florida, said, "I was excited to own the first Tixel in the USA because of the noticeable results accompanied with low downtime – and Tixel has not let me down."
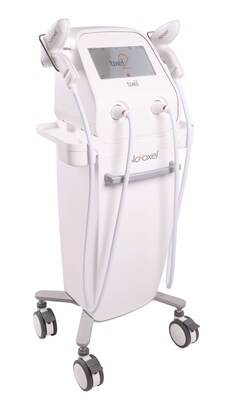
FASTER & EASIER: The new Tixel 2 not only provides the same low-downtime and clinical efficacy as the previous Tixel devices, but it is now faster and easier to use. With two handpieces pre-warmed, clinicians can use the large handpiece and then directly use the smaller handpiece to treat around the eyes or other areas that need more precision. The new "repeat" mode further reduces the length of treatments. The power-on, self-disinfection, and cool-down times are also shorter. Dr. Guy Erlich said, "Because topical anesthesia is not required, it only takes 8 minutes to complete a full-face treatment with Tixel 2. Adding both eyes adds only 2-3 minutes. With all the new Tixel 2 features, treatment time is cut in half."
DIRECT SUPPORT: Novoxel also announces the opening of their US headquarters. Pannell said, "Our employees in the USA will now provide all support for new and previous American customers." Elizabeth Rostan MD, a dermatologist in Charlotte NC, said, "Because the manufacturer established a direct office in the USA, it was a much easier decision to upgrade to Tixel 2. With 2 handpieces, Tixel 2 is going to be an even bigger workhorse in our practice."
To learn more information about the Tixel family of products, please call +1-888-Tixel-US or visit www.Tixel.us.
ABOUT NOVOXEL
Established in 2011, Novoxel Ltd. focuses on scientific and clinical innovation. Novoxel has been granted numerous patents around the world, including USA, EU, UK, Japan, Israel, Korea, and China. The company's Tixel family of products use proprietary Thermo-Mechanical Action (TMA®) to deliver noticeable clinical results even though treatments are non-invasive. There is minimal downtime, no expensive single-use cartridges, no bleeding, and minimal/no pain. Because the affordable treatment is radiation-free, safety eyewear is not required for patients nor clinicians.
Novoxel, Tixel, Tixel 2, Thermo-Mechanical Action, and TMA are all registered trademarks of Novoxel Ltd.
©2024, Novoxel Inc. All rights reserved.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/just-in-time-for-summer-and-loaded-with-new-enhancements-tixel-2-comes-to-the-usa-302164260.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/just-in-time-for-summer-and-loaded-with-new-enhancements-tixel-2-comes-to-the-usa-302164260.html
SOURCE Novoxel Inc.