Koelis, SAS, a leader and innovator in the care of prostate cancer, announced the launch of new products at the prestigious American Urological Association Annual Meeting in New Orleans, LA.
GRENOBLE, France and PRINCETON, N.J., May 11, 2022 (GLOBE NEWSWIRE) -- Koelis, SAS (“Koelis” or the “Company”, www.koelis.com), a leader and innovator in the care of prostate cancer, announced today the launch of new products at the prestigious American Urological Association Annual Meeting in New Orleans, LA.
Koelis will be holding live and interactive demos on its booth #1201 from May 13th to May 15th to demonstrate the exclusive technology and new features of its Trinity 3D imaging and fusion platform.
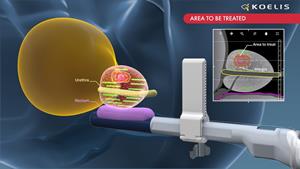
The new products include software for focal cryoablation guidance (“ProMap-FT™”); single-use transperineal biopsy grids (“Perine Grids™”); and MRI processing software (“ProMap Lite™”). Each of these new products complement the Koelis Trinity® MRI-US fusion image guidance system. The Trinity system integrates versatile 3D ultrasound imaging with proprietary MRI-US fusion image guidance that features the Company’s unique prostate motion tracking software (OBT Fusion®). The Koelis Trinity® system is a single, space-efficient system that does not require an interface with an external ultrasound imaging system or external electromagnetic sensors.
Mark Rooney, Koelis’ VP and General Manager in the USA, commented, “We believe that these new product launches are evidence that we are listening to our urologic customers and academic partners who are leading a paradigm shift in prostate cancer care.”
ProMap-FT™ expands the Koelis focal prostate ablation treatment product range to include planning and guidance software for cryoablation. With isotherm specifications on-board, the ProMap-FT software is compatible with each of the two leading vendors of cryoablation systems. Utilizing the Koelis proprietary OBT Fusion® technology, ProMap-FT also has a unique ability to safely and accurately track cryoablation needles to any targeted prostate cancer lesion. Promap-FT also offers versatility in enabling 3D guidance of laser fibers or any other interventional needle.
Single-Use Perine Grids™ includes both “full” and “mini” versions of the Company’s popular reusable transperineal needle guides. By minimizing both cross-contamination and sterilization time and labor, the Koelis single-use Perine Grids will help support the transition of transperineal prostate biopsy to an office-based procedure.
ProMap Lite™ is a software-only version of Koelis MR-Draw™ biopsy planning workstation. Designed to be compatible with PC’s and radiology workstations, ProMap Lite will add flexibility and efficiency to the workflow required to access and process a patient’s MRI prior to his fusion-guide biopsy. ProMap Lite is the first step of the Koelis strategy that ultimately will add AI and cloud-based features to the Trinity system.
Antoine Leroy, Koelis’ Co-founder and Chief Executive Officer, concludes: “Our mission is to innovate continuously to offer prostate biopsy and targeted treatment technology that advance the quality of prostate cancer care. We also seek to offer the highest level of versatility in technology choices to physicians and their patients. We are committed to support accurate and safe prostate interventions, whether these procedures are performed with or without MRI-US fusion, whether transrectal or transperineal, or whether followed-up with active surveillance or treatment.”
About Koelis: Headquartered in Grenoble, France, Koelis has been a pioneer and leader of MRI-US fusion image guidance technology for above 10 years. Featuring proprietary 3D ultrasound and prostate motion tracking software (OBT Fusion®), the Koelis Trinity® system facilitates more accurate biopsy diagnosis as well as enabling “focal” prostate cancer treatment alternatives to traditional “total” organ treatments such as surgical prostatectomy and radiation. The Company’s commitment to minimally invasive prostate cancer treatments includes a multi-center clinical registry (“Violette”, NCT04582656) in Europe based on Trinity-guided, microwave ablation technology.
Koelis: Antoine Leroy, Founder and CEO
e-mail: info@koelis.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/0fff965d-28a1-41a1-8123-1be0c08f44c3