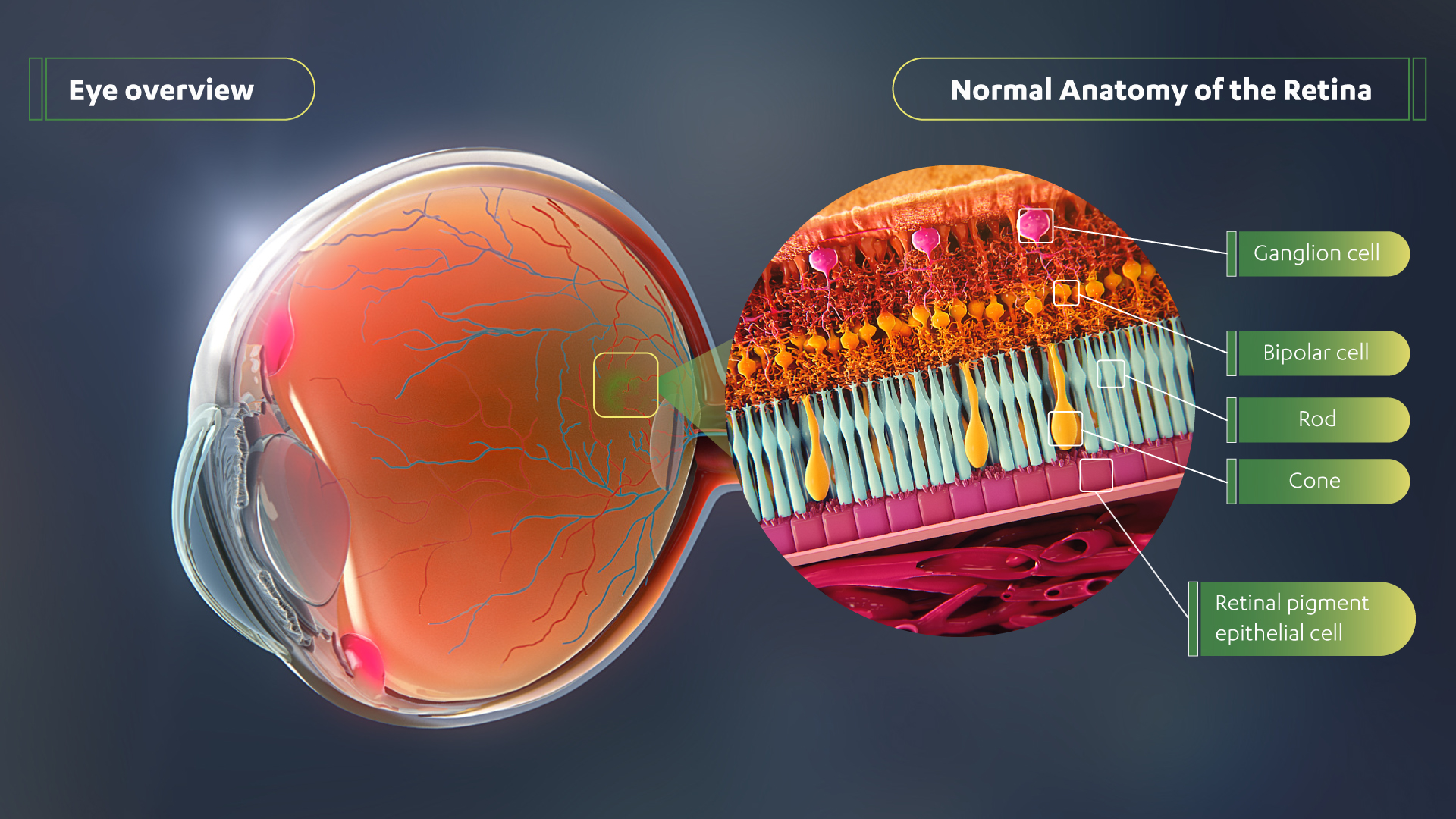
The Janssen Pharmaceutical Companies of Johnson & Johnson announced today new 12-month data from the ongoing Phase 1/2 trial (NCT03252847) of its investigational gene therapy for inherited retinal disease X-linked retinitis pigmentosa (XLRP).
| RARITAN, N.J., Nov. 13, 2020 /PRNewswire/ -- The Janssen Pharmaceutical Companies of Johnson & Johnson announced today new 12-month data from the ongoing Phase 1/2 trial (NCT03252847) of its investigational gene therapy for inherited retinal disease X-linked retinitis pigmentosa (XLRP). The data showed that low and intermediate doses were well-tolerated and continued to demonstrate statistically significant sustained or increased vision improvement across multiple metrics (mean sensitivity, volumetric and pointwise) and modalities (full-field static perimetry and microperimetry). Data on the novel adeno-associated virus retinitis pigmentosa GTPase regulator (AAV-RPGR), jointly developed with MeiraGTx Holdings plc, were presented today as a late-breaking study at the American Academy of Ophthalmology (AAO) 2020 Virtual Annual Meeting. Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8798151-janssen-rpgr-gene-therapy-data-in-patients-with-x-linked-retinitis-pigmentosa/  In patients with XLRP, the photoreceptors that are responsible for converting light into signals that are sent to the brain, function poorly, leading to degeneration of the retina and legal blindness in adulthood. The companies’ AAV-RPGR gene therapy is being investigated for the most common and severe forms of XLRP caused by mutations in the RPGR gene by preserving and improving vision and slowing retinal degeneration. Currently, there are no available treatments. “Living with XLRP is extremely devastating for patients and their families, as there is no treatment available and they live each day knowing they will eventually go blind,” said Michel Michaelides,1 B.Sc., M.B., B.S., M.D. (Res), FRCOphth, FACS, trial investigator, Consultant Ophthalmologist, Moorfields Eye Hospital, Professor of Ophthalmology, University College London. “The continuous upward trend in efficacy we’ve observed through one year with this gene therapy is extremely promising as a potential way to halt the progression toward blindness in these patients.” The primary endpoint of the trial is safety, with secondary endpoints assessing changes in visual function at pre-specified timepoints post-treatment. Baseline values were determined in triplicate. In the dose escalation phase, at 12 months, six of seven patients in the low (n=3) and intermediate (n=4) doses demonstrated improvement or stability in retinal sensitivity in the treated eye as measured by full-field static perimetry and mesopic microperimetry.2
Safety data to date has demonstrated ocular and systemic safety profiles that are anticipated and manageable. The most common adverse events were related to the surgical procedure, transient and resolved without intervention. In the high dose cohort (n=3), inflammation was evident in two of three adults, and measures of visual function were not improved. “We have a great sense of urgency to meet the needs of patients living with XLRP. They need transformational treatment as soon as possible to give them the best chance of maintaining their sight,” said James List, M.D., Ph.D., Global Therapeutic Area Head, Cardiovascular & Metabolism, Janssen Research & Development, LLC. “With our positive 12-month AAV-RPGR data, the stage is now set for advancement into Phase 3, bringing us one step closer to our goal of preserving, and potentially improving, vision for these patients.” Vision-Guided Mobility Data In the nine-month analysis, compared to baseline:
Vision-guided mobility assessment was not carried out in the high dose cohort at the nine-month timepoint due to an implemented protocol revision to align with the dose-expansion cohort assessment schedule. About the Phase 1/2 RPGR Trial (MGT009) and AAV-RPGR The ongoing Phase 1/2 clinical trial consists of three phases: dose-escalation, dose-confirmation and dose-expansion. In the dose-escalation phase (n=10), adults were administered low, intermediate or high dose AAV-RPGR. AAV-RPGR was delivered via subretinal injection targeting the central retina in the eye that was more affected at baseline. The patient’s other eye served as an untreated control. Multiple retinotomies were permitted to enable coverage of the largest possible area of rescuable retina. Perimetry was performed using Octopus 900 full-field static perimetry and MAIA fundus-guided microperimetry, and was conducted at baseline, three, six, nine and 12 months to assess baseline retinal function and change over time. Patients were required to have evidence of relative preservation of retinal structure at the macula and be able to undertake age-appropriate clinical assessments. For more information, visit: https://clinicaltrials.gov/ct2/show/NCT03252847? The European Medicines Agency (EMA) granted PRIME (PRIority MEdicines) and Advanced Therapy Medicinal Product (ATMP) designations to AAV-RPGR to increase interactions and optimize development plans based on data from the ongoing Phase 1/2 clinical trial. The novel AAV-RPGR therapy also received Fast Track designation from the U.S. Food and Drug Administration (FDA) and Orphan designations from the FDA and the EMA. About the Janssen and MeiraGTx Strategic Collaboration About Janssen’s Retinal Portfolio About the Janssen Pharmaceutical Companies of Johnson & Johnson Cautions Concerning Forward-Looking Statements This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding the potential benefits of the collaboration and license agreement with MeiraGTx Holdings plc. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Janssen Research & Development, LLC, any of the other Janssen Pharmaceutical Companies and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; manufacturing difficulties and delays; competition, including technological advances, new products and patents attained by competitors; challenges to patents; product efficacy or safety concerns resulting in product recalls or regulatory action; changes in behavior and spending patterns of purchasers of health care products and services; changes to applicable laws and regulations, including global health care reforms; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended December 29, 2019, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in the company’s subsequent Quarterly Reports on Form 10-Q, and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Neither the Janssen Pharmaceutical Companies nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
1Dr. Michaelides is a scientific founder of, and consultant to, and has a financial relationship with MeiraGTx.
Media Contacts: Jennifer Silvent Investor Relations: Jennifer McIntyre




SOURCE Janssen Pharmaceutical Companies of Johnson & Johnson |




