Levita Magnetics, whose mission is to help more patients get access to better surgery, announced today it has received U.S. Food and Drug Administration (FDA) clearance for its MARS™ platform.
|
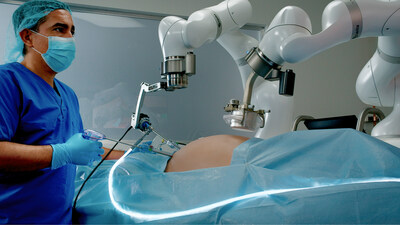
First-of-its-kind minimally invasive surgery platform that combines magnets and machines. MOUNTAIN VIEW, Calif., Aug. 22, 2023 /PRNewswire/ -- Levita Magnetics, whose mission is to help more patients get access to better surgery, announced today it has received U.S. Food and Drug Administration (FDA) clearance for its MARS™ platform. The Levita MARS system is a first-of-its-kind minimally invasive surgical platform aimed at the high-volume abdominal surgery market. Harnessing the power of both magnets and machines, MARS reduces the number of incisions and enables surgeons to have complete control during laparoscopic procedures, all in a compact footprint designed to fit into existing operating rooms. Building on the success of its first commercial product, the Levita Magnetic Surgical System®, Levita designed MARS to deliver the same patient benefits as Magnetic Surgery®, while empowering surgeons with increased control of surgical instruments and providing an efficient way for hospitals and ambulatory surgery centers (ASCs) to incorporate this new technology. "Today marks a significant milestone in Levita's mission to provide more patients access to state-of-the-art surgical technology. MARS is poised to revolutionize surgical options for a broad range of patients," said Dr. Alberto Rodriguez-Navarro, surgeon, founder, and CEO of Levita Magnetics. "With this FDA clearance, we eagerly anticipate making a substantial impact across the value chain." MARS aims to deliver triple impact:
"MARS by Levita has the potential to reshape the surgical industry and forever change medical innovation. Our pioneering MARS platform gives patients and surgeons a transformative tool that will usher in a fundamental shift in surgery for years to come," said Levita Chairperson Maria Sainz. "Levita's system can aid in surgeon proficiency and efficiency, and can reduce the need for assistive personnel, signaling a major achievement not only for Levita but for surgical care." For more information on Levita Magnetics and MARS please visit levita.com. About Levita Media contact:
SOURCE Levita |