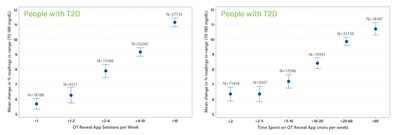
LifeScan, today announced the presentation of new real-world evidence from patient-generated data showing significantly improved readings in range and lower mean glucose in people using the OneTouch Verio Flex® blood glucose meter with the OneTouch Reveal® mobile application – the late-breaking abstract was presented at the 82nd Scientific Sessions of the American Diabetes Association (ADA) in New Orleans (Abstract Poster 60-LB).
MALVERN, Pa., June 6, 2022 /PRNewswire/ -- LifeScan, a world leader in blood glucose monitoring, today announced the presentation of new real-world evidence from patient-generated data showing significantly improved readings in range and lower mean glucose in people using the OneTouch Verio Flex® blood glucose meter with the OneTouch Reveal® mobile application – the late-breaking abstract was presented at the 82nd Scientific Sessions of the American Diabetes Association (ADA) in New Orleans (Abstract Poster 60-LB). “The insights provided through our retrospective analysis of patient-generated real-world data demonstrate a significant improvement in blood glucose readings in range in those using the OneTouch Verio Flex meter and OneTouch Reveal app,” said Dr. Elizabeth Holt, Head of Global Medical, Clinical, and Safety, LifeScan. “These improved readings in range findings are important as 95% of people with diabetes who rely on glucose testing use a blood glucose meter.” Researchers examined anonymized blood glucose readings with app analytics from a LifeScan data-lake of 100,650 people with type 2 diabetes (T2D) and 20,324 people with type 1 diabetes (T1D), comparing data from their first 14 days to 14 days prior to a 90-day timepoint using paired within-subject differences. Important findings from the study include:
About LifeScan
SOURCE Lifescan, Inc. |