LIPOGEMS, an innovative medtech company with a leading and propriety adipose tissue system technology announces that it has achieve a major milestone of 35,000 procedures globally.
NORCROSS, Ga., June 11, 2019 /PRNewswire/ -- LIPOGEMS, an innovative medtech company with a leading and propriety adipose tissue system technology announces today that it has achieve a major milestone of 35,000 procedures globally.
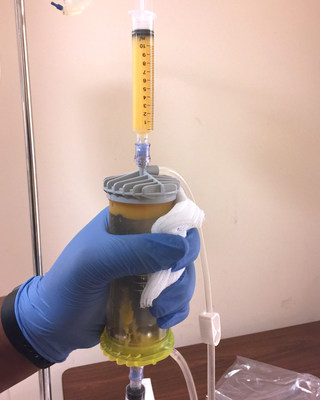
LIPOGEMS is leading the way by providing orthopedic specialties an option for the "treatment gap" between conservative therapies (physical therapy, corticosteroids, viscosupplementation) and major, surgical intervention. The LIPOGEMS® system provides patients a natural, minimally invasive, FDA cleared option that is offered by top orthopaedic physicians and institutions from around the world.
With LIPOGEMS' FDA clearance and strong scientific evidence, the goal of the company is to offer a compliant and reliable option that may help thousands with orthopaedic issues. LIPOGEMS has expanded into 28 countries worldwide and through rapid adoption from the top orthopedic institutions worldwide, LIPOGEMS achieved this major milestone responsibly.
Orthopaedic physicians utilize the technology because it is FDA cleared and can be easily incorporated into their orthopaedic practice.
- Simple, Minimally invasive procedure in the office/procedure room: Efficient processing and procedure that can be performed in less than an hour
- Added to orthopaedic or arthroscopic surgery to provide cushion and support for the healing process
Utilizing its patented LIPOGEMS System, LIPOGEMS provides a valuable option for patients and physicians.
- FDA Compliant: Meets guidelines for minimal manipulation and intended for homologous use
- High Quality Tissue that Does Not Decline with Age: Adipose tissue contains a high concentration of reparative cells1 that do not decline with age2,3,4 and the processing preserves the cell and tissue microarchitecture
- Minimal Risk of Rejection or Infection: Uses the patient's own adipose tissue
- Comprehensive Kit: Disposable kit containing materials for harvesting and processing of the adipose tissue
- Pure: Only uses saline to wash, rinse the tissue
- Economical: No centrifuge or capital equipment needed
- Sterile: Closed-loop system reduces risk of contamination
- Optimal size for injecting into multiple areas in a single setting
Carl Llewellyn, CEO for LIPOGEMS USA comments: "We're extremely proud of our ability to reach this milestone by partnering with leading orthopaedic physicians and scientists, both domestically and internationally, to provide an FDA cleared, simple and efficient option for patients using their own fat (adipose tissue). Reaching 35,000 procedures is a testament to the market acceptance of adipose tissue and the LIPOGEMS technology. We thank our physician partners who have utilized the LIPOGEMS device since its launch in 2015 and who see it as a consistent and reliable solution for their patients. Most importantly, we are grateful to have touched the lives and positively impacted the patients that have received LIPOGEMS."
References:
- Caplan A: 2013: Mesenchymal stem cells environmentally responsive therapeutics for regenerative medicine.Experimental & Molecular medicine. 45(11):e54.
- Beane, O.S., et al. (2014). "Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/ stromal cells." PloS one 9.12. e115963.
- Stolzing, A., et al. (2008). "Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies." Mechanisms of aging and development 129.3: 163-173.
- Kern, S., et al. (2006). "Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue." Stem cells 24.5: 1294-1301

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/lipogems-announces-milestone-of-35-000-procedures-performed-for-patients-between-conservative-therapy-and-major-invasive-surgery-300865577.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/lipogems-announces-milestone-of-35-000-procedures-performed-for-patients-between-conservative-therapy-and-major-invasive-surgery-300865577.html
SOURCE LIPOGEMS




