Malignant Mesothelioma Market Outlook 2024-2034:
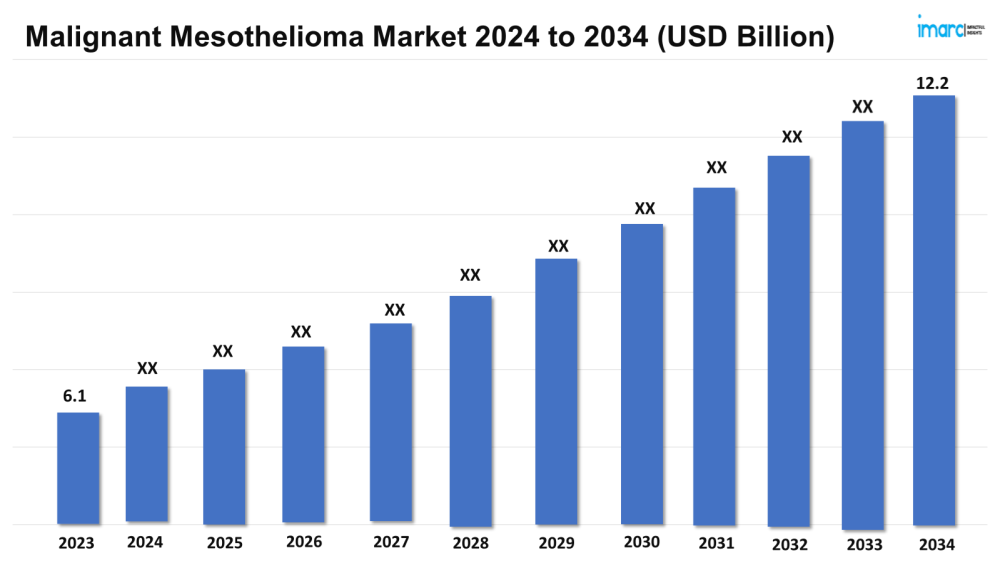
The malignant mesothelioma market size reached a value of US$ 6.1 Billion in 2023. Looking forward, the market is expected to reach US$ 12.2 Billion by 2034, exhibiting a growth rate (CAGR) of 6.5% during 2024-2034. The market is driven by the escalating demand for treatments like pembrolizumab and nivolumab that offer improved patient outcomes. Additionally, there's an increasing focus on early detection methods using biomarkers and advanced imaging techniques to enhance diagnosis accuracy.
Advancements of Immunotherapy Treatments: Driving the Malignant Mesothelioma Market
One of the most notable immunotherapy drugs for mesothelioma is pembrolizumab (Keytruda), a checkpoint inhibitor that targets the PD-1 pathway. Pembrolizumab has demonstrated significant efficacy in extending survival and improving the quality of life for mesothelioma patients. Studies have shown that patients treated with pembrolizumab have experienced durable responses and longer progression-free survival compared to those receiving conventional therapies. This success has led to its approval by regulatory agencies and widespread adoption in clinical practice, making it a cornerstone of modern mesothelioma treatment. Another key player in the immunotherapy landscape is nivolumab, which, like pembrolizumab, is a PD-1 inhibitor. Nivolumab has been evaluated in various clinical trials for mesothelioma and has shown promising results, particularly when used in combination with other immunotherapy agents such as ipilimumab, a CTLA-4 inhibitor.
Request a PDF Sample Report: https://www.imarcgroup.com/malignant-mesothelioma-market/requestsample
This combination therapy has been found to enhance the anti-tumor immune response, leading to better clinical outcomes. The FDA’s approval of the nivolumab and ipilimumab combination for mesothelioma treatment underscores the growing importance of immunotherapy in this field. Furthermore, the development of personalized immunotherapy approaches is gaining momentum. Advances in genetic and molecular profiling of tumors allow for the identification of specific biomarkers that can predict response to immunotherapy. This precision medicine approach ensures that patients receive treatments that are most likely to be effective for their particular cancer subtype, thereby optimizing therapeutic outcomes and minimizing unnecessary side effects. The growing body of evidence supporting the efficacy of immunotherapy has also spurred increased investment in research and development. Pharmaceutical companies and research institutions are conducting numerous clinical trials to explore new immunotherapeutic agents and combinations. This robust pipeline of investigational therapies promises to further expand the treatment options available for mesothelioma patients in the near future.
Increasing Demand for Personalized Medicine: Contributing to Market Expansion
The market is being significantly transformed by the advent of personalized medicine, which tailors treatment to the genetic profile of individual patients. This approach is rooted in the understanding that each tumor has a unique genetic and molecular makeup, and leveraging this knowledge allows for more precise and effective therapies. Personalized medicine is driving advancements in the diagnosis, treatment, and overall management of mesothelioma, leading to improved patient outcomes and survival rates. One of the key components of personalized medicine in mesothelioma is the use of genetic and molecular profiling. Techniques such as next-generation sequencing (NGS) enable detailed analysis of the genetic mutations and alterations present in a patient’s tumor. By identifying specific biomarkers, clinicians can predict how the tumor will respond to certain treatments and select the most appropriate therapy, thereby stimulating the market.
Targeted therapies are a cornerstone of personalized medicine. Unlike traditional chemotherapy, which indiscriminately attacks rapidly dividing cells, targeted therapies are designed to interfere with specific molecular pathways that are essential for tumor growth and survival. Drugs such as bevacizumab, which inhibits angiogenesis, and selumetinib, which targets the MAPK/ERK pathway, have shown promise in clinical trials for mesothelioma patients with specific genetic profiles. These therapies offer the potential for greater efficacy and reduced toxicity compared to conventional treatments. Another significant aspect of personalized medicine is the development of immunotherapies tailored to the patient’s immune landscape. By understanding the tumor’s interaction with the immune system, treatments can be designed to enhance the body’s immune response against cancer cells. For example, PD-1 and CTLA-4 inhibitors, such as pembrolizumab and nivolumab, have been used in combination with other agents to boost their effectiveness in mesothelioma patients with high PD-L1 expression. This personalized approach to immunotherapy has led to remarkable improvements in patient outcomes.
Focus on Early Detection:
Early detection is increasingly driving the malignant mesothelioma market, significantly enhancing the potential for successful treatment outcomes and extending patient survival rates. Mesothelioma, a cancer linked primarily to asbestos exposure, is notoriously difficult to diagnose in its early stages due to its long latency period and non-specific symptoms. One of the key advancements in early detection is the development and utilization of biomarkers. In mesothelioma, specific biomarkers such as soluble mesothelin-related peptides (SMRP), fibulin-3, and osteopontin have shown promise in aiding early diagnosis. Another significant innovation is the use of advanced imaging techniques. High-resolution computed tomography (HRCT) and positron emission tomography (PET) scans are increasingly being used to detect mesothelioma at earlier stages. Additionally, magnetic resonance imaging (MRI) is being explored for its potential to offer even greater detail in certain types of mesothelioma, such as peritoneal mesothelioma.
Liquid biopsies represent another frontier in early detection. This cutting-edge technology involves analyzing circulating tumor DNA (ctDNA) in a patient’s blood, which can reveal the presence of cancerous cells long before symptoms arise. Liquid biopsies are less invasive than traditional tissue biopsies and can be repeated frequently, providing ongoing monitoring of disease progression and treatment response. This early and continuous detection capability is poised to impact the management and prognosis of mesothelioma significantly. The integration of artificial intelligence (AI) and machine learning in early detection is also noteworthy. AI algorithms can analyze vast amounts of medical data, including imaging scans and genetic information, to identify patterns and predict the presence of mesothelioma with high accuracy. These technologies are enhancing the speed and precision of diagnosis, enabling earlier intervention and personalized treatment plans tailored to the specific characteristics of the patient’s cancer.
Buy Full Report: https://www.imarcgroup.com/checkout?id=9205&method=587
Leading Companies in the Malignant Mesothelioma Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global malignant mesothelioma market, several leading companies are at the forefront of technological advancements and application development. Some of the major players include Novocure, Bristol-Myers Squibb, and Merck & Co., Inc. These companies are investing in R&D activities to introduce novel treatment options.
Novocure announced positive results from its STELLAR Phase 2 trial, demonstrating that TTFields, in combination with standard chemotherapy, significantly improved overall survival rates for mesothelioma patients. This breakthrough has solidified Novocure's position as a leader in non-invasive cancer treatments, offering new hope for patients with this challenging disease.
Bristol-Myers Squibb is a major player in the malignant mesothelioma market, largely due to its development of immune checkpoint inhibitors. The FDA has approved the combination of nivolumab (Opdivo) and ipilimumab (Yervoy) for the first-line treatment of unresectable malignant pleural mesothelioma.
Merck & Co., Inc. is another key player in the mesothelioma market with its flagship immunotherapy drug, pembrolizumab (Keytruda). Pembrolizumab is being extensively studied for its efficacy in treating mesothelioma.
Request for customization: https://www.imarcgroup.com/request?type=report&id=9205&flag=E
Regional Analysis:
The major markets for malignant mesothelioma include the United States, Germany, Spain, Italy, France, the United Kingdom, and Japan. According to projections by IMARC, the United States has the largest patient pool for malignant mesothelioma while also representing the biggest market for its treatment. This can be attributed to the introduction of innovative therapies and advanced diagnostic technologies.
Moreover, increased public health initiatives and awareness campaigns about the dangers of asbestos exposure are also influencing the malignant mesothelioma market in the United States. These efforts are crucial in promoting regular screenings and early detection, especially among high-risk populations such as construction workers and veterans. Enhanced awareness leads to earlier diagnosis and better management of the disease, ultimately improving patient outcomes across the country.
Besides this, leading pharmaceutical companies such as Novocure, Bristol-Myers Squibb, and Merck are at the forefront of this transformation. Novocure's Tumor Treating Fields (TTFields) therapy has shown promise in improving survival rates when combined with standard chemotherapy, as evidenced by positive results from their STELLAR Phase 2 trial. Bristol-Myers Squibb's immune checkpoint inhibitors, nivolumab (Opdivo) and ipilimumab (Yervoy) have received FDA approval for first-line treatment of unresectable malignant pleural mesothelioma, based on significant survival benefits demonstrated in the CheckMate-743 trial. Additionally, Merck's pembrolizumab (Keytruda) continues to show durable responses in advanced mesothelioma patients, particularly those with high PD-L1 expression, according to the KEYNOTE-158 trial findings.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the malignant mesothelioma market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the malignant mesothelioma market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current malignant mesothelioma marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/malignant-mesothelioma-market
IMARC Group Offer Other Reports:
Postmenopausal Vaginal Atrophy Market: The 7 major postmenopausal vaginal atrophy market reached a value of US$ 1.6 Billion in 2023, and projected the 7MM to reach S$ 2.7 Billion by 2034, exhibiting a growth rate (CAGR) of 5.04% during the forecast period from 2024 to 2034.
Seasonal Influenza Market: The 7 major seasonal influenza market reached a value of US$ 8.9 Billion in 2023, and projected the 7MM to reach US$ 41.6 Billion by 2034, exhibiting a growth rate (CAGR) of 15.08% during the forecast period from 2024 to 2034.
Metastatic Melanoma Market: The 7 major metastatic melanoma market is expected to exhibit a CAGR of 8.23% during the forecast period from 2024 to 2034.
Lennox-Gastaut Syndrome Market: The 7 major lennox-gastaut syndrome market is expected to exhibit a CAGR of 4.63% during the forecast period from 2024 to 2034.
Chemotherapy-Induced Peripheral Neuropathy Market: The 7 major chemotherapy-induced peripheral neuropathy market is expected to exhibit a CAGR of 4.44% during the forecast period from 2024 to 2034.
Bacterial Conjunctivitis Market: The 7 major bacterial conjunctivitis market reached a value of US$ 3.0 Billion in 2023, and projected the 7MM to reach US$ 4.3 Billion by 2034, exhibiting a growth rate (CAGR) of 3.23% during the forecast period from 2024 to 2034.
Rotavirus Market: The 7 major rotavirus market is expected to exhibit a CAGR of 8.62% during the forecast period from 2024-2034.
Plantar Fasciitis Market: The 7 major plantar fasciitis market is expected to exhibit a CAGR of 17.88% during the forecast period from 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The malignant mesothelioma market size reached a value of US$ 6.1 Billion in 2023. Looking forward, the market is expected to reach US$ 12.2 Billion by 2034, exhibiting a growth rate (CAGR) of 6.5% during 2024-2034. The market is driven by the escalating demand for treatments like pembrolizumab and nivolumab that offer improved patient outcomes. Additionally, there's an increasing focus on early detection methods using biomarkers and advanced imaging techniques to enhance diagnosis accuracy.
Advancements of Immunotherapy Treatments: Driving the Malignant Mesothelioma Market
One of the most notable immunotherapy drugs for mesothelioma is pembrolizumab (Keytruda), a checkpoint inhibitor that targets the PD-1 pathway. Pembrolizumab has demonstrated significant efficacy in extending survival and improving the quality of life for mesothelioma patients. Studies have shown that patients treated with pembrolizumab have experienced durable responses and longer progression-free survival compared to those receiving conventional therapies. This success has led to its approval by regulatory agencies and widespread adoption in clinical practice, making it a cornerstone of modern mesothelioma treatment. Another key player in the immunotherapy landscape is nivolumab, which, like pembrolizumab, is a PD-1 inhibitor. Nivolumab has been evaluated in various clinical trials for mesothelioma and has shown promising results, particularly when used in combination with other immunotherapy agents such as ipilimumab, a CTLA-4 inhibitor.
Request a PDF Sample Report: https://www.imarcgroup.com/malignant-mesothelioma-market/requestsample
This combination therapy has been found to enhance the anti-tumor immune response, leading to better clinical outcomes. The FDA’s approval of the nivolumab and ipilimumab combination for mesothelioma treatment underscores the growing importance of immunotherapy in this field. Furthermore, the development of personalized immunotherapy approaches is gaining momentum. Advances in genetic and molecular profiling of tumors allow for the identification of specific biomarkers that can predict response to immunotherapy. This precision medicine approach ensures that patients receive treatments that are most likely to be effective for their particular cancer subtype, thereby optimizing therapeutic outcomes and minimizing unnecessary side effects. The growing body of evidence supporting the efficacy of immunotherapy has also spurred increased investment in research and development. Pharmaceutical companies and research institutions are conducting numerous clinical trials to explore new immunotherapeutic agents and combinations. This robust pipeline of investigational therapies promises to further expand the treatment options available for mesothelioma patients in the near future.
Increasing Demand for Personalized Medicine: Contributing to Market Expansion
The market is being significantly transformed by the advent of personalized medicine, which tailors treatment to the genetic profile of individual patients. This approach is rooted in the understanding that each tumor has a unique genetic and molecular makeup, and leveraging this knowledge allows for more precise and effective therapies. Personalized medicine is driving advancements in the diagnosis, treatment, and overall management of mesothelioma, leading to improved patient outcomes and survival rates. One of the key components of personalized medicine in mesothelioma is the use of genetic and molecular profiling. Techniques such as next-generation sequencing (NGS) enable detailed analysis of the genetic mutations and alterations present in a patient’s tumor. By identifying specific biomarkers, clinicians can predict how the tumor will respond to certain treatments and select the most appropriate therapy, thereby stimulating the market.
Targeted therapies are a cornerstone of personalized medicine. Unlike traditional chemotherapy, which indiscriminately attacks rapidly dividing cells, targeted therapies are designed to interfere with specific molecular pathways that are essential for tumor growth and survival. Drugs such as bevacizumab, which inhibits angiogenesis, and selumetinib, which targets the MAPK/ERK pathway, have shown promise in clinical trials for mesothelioma patients with specific genetic profiles. These therapies offer the potential for greater efficacy and reduced toxicity compared to conventional treatments. Another significant aspect of personalized medicine is the development of immunotherapies tailored to the patient’s immune landscape. By understanding the tumor’s interaction with the immune system, treatments can be designed to enhance the body’s immune response against cancer cells. For example, PD-1 and CTLA-4 inhibitors, such as pembrolizumab and nivolumab, have been used in combination with other agents to boost their effectiveness in mesothelioma patients with high PD-L1 expression. This personalized approach to immunotherapy has led to remarkable improvements in patient outcomes.
Focus on Early Detection:
Early detection is increasingly driving the malignant mesothelioma market, significantly enhancing the potential for successful treatment outcomes and extending patient survival rates. Mesothelioma, a cancer linked primarily to asbestos exposure, is notoriously difficult to diagnose in its early stages due to its long latency period and non-specific symptoms. One of the key advancements in early detection is the development and utilization of biomarkers. In mesothelioma, specific biomarkers such as soluble mesothelin-related peptides (SMRP), fibulin-3, and osteopontin have shown promise in aiding early diagnosis. Another significant innovation is the use of advanced imaging techniques. High-resolution computed tomography (HRCT) and positron emission tomography (PET) scans are increasingly being used to detect mesothelioma at earlier stages. Additionally, magnetic resonance imaging (MRI) is being explored for its potential to offer even greater detail in certain types of mesothelioma, such as peritoneal mesothelioma.
Liquid biopsies represent another frontier in early detection. This cutting-edge technology involves analyzing circulating tumor DNA (ctDNA) in a patient’s blood, which can reveal the presence of cancerous cells long before symptoms arise. Liquid biopsies are less invasive than traditional tissue biopsies and can be repeated frequently, providing ongoing monitoring of disease progression and treatment response. This early and continuous detection capability is poised to impact the management and prognosis of mesothelioma significantly. The integration of artificial intelligence (AI) and machine learning in early detection is also noteworthy. AI algorithms can analyze vast amounts of medical data, including imaging scans and genetic information, to identify patterns and predict the presence of mesothelioma with high accuracy. These technologies are enhancing the speed and precision of diagnosis, enabling earlier intervention and personalized treatment plans tailored to the specific characteristics of the patient’s cancer.
Buy Full Report: https://www.imarcgroup.com/checkout?id=9205&method=587
Leading Companies in the Malignant Mesothelioma Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global malignant mesothelioma market, several leading companies are at the forefront of technological advancements and application development. Some of the major players include Novocure, Bristol-Myers Squibb, and Merck & Co., Inc. These companies are investing in R&D activities to introduce novel treatment options.
Novocure announced positive results from its STELLAR Phase 2 trial, demonstrating that TTFields, in combination with standard chemotherapy, significantly improved overall survival rates for mesothelioma patients. This breakthrough has solidified Novocure's position as a leader in non-invasive cancer treatments, offering new hope for patients with this challenging disease.
Bristol-Myers Squibb is a major player in the malignant mesothelioma market, largely due to its development of immune checkpoint inhibitors. The FDA has approved the combination of nivolumab (Opdivo) and ipilimumab (Yervoy) for the first-line treatment of unresectable malignant pleural mesothelioma.
Merck & Co., Inc. is another key player in the mesothelioma market with its flagship immunotherapy drug, pembrolizumab (Keytruda). Pembrolizumab is being extensively studied for its efficacy in treating mesothelioma.
Request for customization: https://www.imarcgroup.com/request?type=report&id=9205&flag=E
Regional Analysis:
The major markets for malignant mesothelioma include the United States, Germany, Spain, Italy, France, the United Kingdom, and Japan. According to projections by IMARC, the United States has the largest patient pool for malignant mesothelioma while also representing the biggest market for its treatment. This can be attributed to the introduction of innovative therapies and advanced diagnostic technologies.
Moreover, increased public health initiatives and awareness campaigns about the dangers of asbestos exposure are also influencing the malignant mesothelioma market in the United States. These efforts are crucial in promoting regular screenings and early detection, especially among high-risk populations such as construction workers and veterans. Enhanced awareness leads to earlier diagnosis and better management of the disease, ultimately improving patient outcomes across the country.
Besides this, leading pharmaceutical companies such as Novocure, Bristol-Myers Squibb, and Merck are at the forefront of this transformation. Novocure's Tumor Treating Fields (TTFields) therapy has shown promise in improving survival rates when combined with standard chemotherapy, as evidenced by positive results from their STELLAR Phase 2 trial. Bristol-Myers Squibb's immune checkpoint inhibitors, nivolumab (Opdivo) and ipilimumab (Yervoy) have received FDA approval for first-line treatment of unresectable malignant pleural mesothelioma, based on significant survival benefits demonstrated in the CheckMate-743 trial. Additionally, Merck's pembrolizumab (Keytruda) continues to show durable responses in advanced mesothelioma patients, particularly those with high PD-L1 expression, according to the KEYNOTE-158 trial findings.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the malignant mesothelioma market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the malignant mesothelioma market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current malignant mesothelioma marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/malignant-mesothelioma-market
IMARC Group Offer Other Reports:
Postmenopausal Vaginal Atrophy Market: The 7 major postmenopausal vaginal atrophy market reached a value of US$ 1.6 Billion in 2023, and projected the 7MM to reach S$ 2.7 Billion by 2034, exhibiting a growth rate (CAGR) of 5.04% during the forecast period from 2024 to 2034.
Seasonal Influenza Market: The 7 major seasonal influenza market reached a value of US$ 8.9 Billion in 2023, and projected the 7MM to reach US$ 41.6 Billion by 2034, exhibiting a growth rate (CAGR) of 15.08% during the forecast period from 2024 to 2034.
Metastatic Melanoma Market: The 7 major metastatic melanoma market is expected to exhibit a CAGR of 8.23% during the forecast period from 2024 to 2034.
Lennox-Gastaut Syndrome Market: The 7 major lennox-gastaut syndrome market is expected to exhibit a CAGR of 4.63% during the forecast period from 2024 to 2034.
Chemotherapy-Induced Peripheral Neuropathy Market: The 7 major chemotherapy-induced peripheral neuropathy market is expected to exhibit a CAGR of 4.44% during the forecast period from 2024 to 2034.
Bacterial Conjunctivitis Market: The 7 major bacterial conjunctivitis market reached a value of US$ 3.0 Billion in 2023, and projected the 7MM to reach US$ 4.3 Billion by 2034, exhibiting a growth rate (CAGR) of 3.23% during the forecast period from 2024 to 2034.
Rotavirus Market: The 7 major rotavirus market is expected to exhibit a CAGR of 8.62% during the forecast period from 2024-2034.
Plantar Fasciitis Market: The 7 major plantar fasciitis market is expected to exhibit a CAGR of 17.88% during the forecast period from 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800